Fipronil Triggers Immunotoxicity Through Reactive Oxygen Species-Driven Mitochondrial Apoptosis in Thymocytes
- PMID: 40137531
- PMCID: PMC11945543
- DOI: 10.3390/toxics13030204
Fipronil Triggers Immunotoxicity Through Reactive Oxygen Species-Driven Mitochondrial Apoptosis in Thymocytes
Abstract
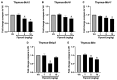
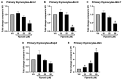
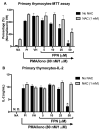
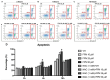
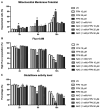
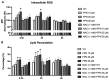
Fipronil (FPN), a widely used pesticide, is associated with significant immunotoxic effects, particularly impacting thymocyte survival and immune homeostasis. This study explores the mechanistic pathways underlying FPN-induced apoptosis and oxidative stress. Short-term FPN exposure (1-10 mg/kg) notably suppressed the expression of both anti-apoptotic (Bcl-2, Bcl-6, Mcl-1) and pro-apoptotic (Bnip3, Bim) genes in thymic tissues in vivo. Additionally, in isolated primary thymocytes, FPN directly decreased the expression of Bcl-2, Bcl-6, Mcl-1, and Bnip3 expression, coupled with a significant increase in pro-apoptotic Bim expression in a dose-dependent manner. FPN treatment directly led to elevated reactive oxygen species (ROS), lipid peroxidation, mitochondrial membrane depolarization, reduced cellular metabolic activity, and depleted intracellular calcium and glutathione (GSH) levels, indicating mitochondrial dysfunction and oxidative stress. Annexin V/PI staining confirmed that FPN induced late-stage apoptosis and necrosis in primary thymocytes. These findings elucidate the immunotoxic effects of FPN on thymocytes, highlighting its detrimental impact on immune system integrity, thymic development, and T cell maturation through oxidative damage and mitochondrial-mediated apoptosis.
Keywords: BCL-2 family; apoptosis; fipronil; glutathione; immunotoxicity; lipid peroxidation; mitochondrial membrane potential; reactive oxygen species.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Induction of Thymus Atrophy and Disruption of Thymocyte Development by Fipronil through Dysregulation of IL-7-Associated Genes.Chem Res Toxicol. 2024 Sep 16;37(9):1488-1500. doi: 10.1021/acs.chemrestox.4c00060. Epub 2024 Aug 14. Chem Res Toxicol. 2024. PMID: 39141674 Free PMC article.
-
Fipronil induces apoptosis and cell cycle arrest in porcine oocytes during in vitro maturation.Apoptosis. 2019 Oct;24(9-10):718-729. doi: 10.1007/s10495-019-01552-w. Apoptosis. 2019. PMID: 31240517
-
Reactive oxygen species and mitogen-activated protein kinase induce apoptotic death of SH-SY5Y cells in response to fipronil.Toxicol Lett. 2012 May 20;211(1):18-28. doi: 10.1016/j.toxlet.2012.02.022. Epub 2012 Mar 7. Toxicol Lett. 2012. PMID: 22421270
-
Luteolin attenuates Fipronil-induced neurotoxicity through reduction of the ROS-mediated oxidative stress in rat brain mitochondria.Pestic Biochem Physiol. 2021 Mar;173:104785. doi: 10.1016/j.pestbp.2021.104785. Epub 2021 Jan 21. Pestic Biochem Physiol. 2021. PMID: 33771263
-
Mitochondrial Oxidative Stress Is the General Reason for Apoptosis Induced by Different-Valence Heavy Metals in Cells and Mitochondria.Int J Mol Sci. 2023 Sep 22;24(19):14459. doi: 10.3390/ijms241914459. Int J Mol Sci. 2023. PMID: 37833908 Free PMC article. Review.
References
-
- Mohamed F., Senarathna L., Percy A., Abeyewardene M., Eaglesham G., Cheng R., Azher S., Hittarage A., Dissanayake W., Sheriff M.R., et al. Acute Human Self-Poisoning with the N-Phenylpyrazole Insecticide Fipronil–A GABAA-Gated Chloride Channel Blocker. J. Toxicol. Clin. Toxicol. 2004;42:955–963. doi: 10.1081/CLT-200041784. - DOI - PMC - PubMed
-
- Environmental Fate and Toxicology of Fipronil. [(accessed on 24 January 2025)]. Available online: https://www.jstage.jst.go.jp/article/jpestics/32/3/32_3_189/_article?for....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

