Distinguishing Low Expression Levels of Human Epidermal Growth Factor Receptor 2 in Breast Cancer: Insights from Qualitative and Quantitative Magnetic Resonance Imaging Analysis
- PMID: 40137571
- PMCID: PMC11945706
- DOI: 10.3390/tomography11030031
Distinguishing Low Expression Levels of Human Epidermal Growth Factor Receptor 2 in Breast Cancer: Insights from Qualitative and Quantitative Magnetic Resonance Imaging Analysis
Abstract
Background: The discovery of novel antibody-drug conjugates for low-expression human epidermal growth factor receptor 2 (HER2-low) breast cancer highlights the inadequacy of the conventional binary classification of HER2 status as either negative or positive. Identification of HER2-low breast cancer is crucial for selecting patients who may benefit from targeted therapies. This study aims to determine whether qualitative and quantitative magnetic resonance imaging (MRI) features can effectively reflect low-HER2-expression breast cancer.
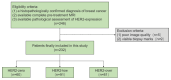
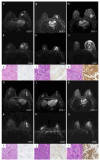
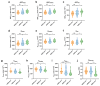
Methods: Pre-treatment breast MRI images from 232 patients with pathologically confirmed breast cancer were retrospectively analyzed. Both clinicopathologic and MRI features were recorded. Qualitative MRI features included Breast Imaging Reporting and Data System (BI-RADS) descriptors from dynamic contrast-enhanced MRI (DCE-MRI), as well as intratumoral T2 hyperintensity and peritumoral edema observed in T2-weighted imaging (T2WI). Quantitative features were derived from diffusion kurtosis imaging (DKI) using multiple b-values and included statistics such as mean, median, 5th and 95th percentiles, skewness, kurtosis, and entropy from apparent diffusion coefficient (ADC), Dapp, and Kapp histograms. Differences in clinicopathologic, qualitative, and quantitative MRI features were compared across groups, with multivariable logistic regression used to identify significant independent predictors of HER2-low breast cancer. The discriminative power of MRI features was assessed using receiver operating characteristic (ROC) curves.
Results: HER2 status was categorized as HER2-zero (n = 60), HER2-low (n = 91), and HER2-overexpressed (n = 81). Clinically, estrogen receptor (ER), progesterone receptor (PR), hormone receptor (HR), and Ki-67 levels significantly differed between the HER2-low group and others (all p < 0.001). In MRI analyses, intratumoral T2 hyperintensity was more prevalent in HER2-low cases (p = 0.009, p = 0.008). Mass lesions were more common in the HER2-zero group than in the HER2-low group (p = 0.038), and mass shape (p < 0.001) and margin (p < 0.001) significantly varied between the HER2 groups, with mass shape emerging as an independent predictive factor (HER2-low vs. HER2-zero: p = 0.010, HER2-low vs. HER2-over: p = 0.012). Qualitative MRI features demonstrated an area under the curve (AUC) of 0.763 (95% confidence interval [CI]: 0.667-0.859) for distinguishing HER2-low from HER2-zero status. Quantitative features showed distinct differences between HER2-low and HER2-overexpression groups, particularly in non-mass enhancement (NME) lesions. Combined variables achieved the highest predictive accuracy for HER2-low status, with an AUC of 0.802 (95% CI: 0.701-0.903).
Conclusions: Qualitative and quantitative MRI features offer valuable insights into low-HER2-expression breast cancer. While qualitative features are more effective for mass lesions, quantitative features are more suitable for NME lesions. These findings provide a more accessible and cost-effective approach to noninvasively identifying patients who may benefit from targeted therapy.
Keywords: breast cancer; human epidermal growth factor receptor 2; low HER2 expression; magnetic resonance imaging.
Conflict of interest statement
Author Caixia Fu was employed by the company Siemens Healthcare. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Combining conventional magnetic resonance imaging (MRI) parameters with clinicopathologic data for differentiation of the three-tiered human epidermal growth factor receptor 2 (HER2) status in breast cancer.Clin Radiol. 2025 Jul;86:106955. doi: 10.1016/j.crad.2025.106955. Epub 2025 May 14. Clin Radiol. 2025. PMID: 40479855
-
Quantitative Parameters of Intravoxel Incoherent Movement Imaging and Dynamic Contrast Enhancement MRI for the Prediction of HER2-Zero, -Low, and -Positive Breast Cancers.Acad Radiol. 2025 Apr;32(4):1851-1860. doi: 10.1016/j.acra.2024.11.011. Epub 2024 Nov 26. Acad Radiol. 2025. PMID: 39592385
-
Preoperative Differentiation of HER2-Zero and HER2-Low from HER2-Positive Invasive Ductal Breast Cancers Using BI-RADS MRI Features and Machine Learning Modeling.J Magn Reson Imaging. 2025 Feb;61(2):928-941. doi: 10.1002/jmri.29447. Epub 2024 May 10. J Magn Reson Imaging. 2025. PMID: 38726477 Free PMC article.
-
Improved Differential Diagnosis Based on BI-RADS Descriptors and Apparent Diffusion Coefficient for Breast Lesions: A Multiparametric MRI Analysis as Compared to Kaiser Score.Acad Radiol. 2023 Sep;30 Suppl 2:S93-S103. doi: 10.1016/j.acra.2023.03.035. Epub 2023 May 24. Acad Radiol. 2023. PMID: 37236897 Review.
-
Role of breast MRI BI-RADS descriptors in discrimination of non-mass enhancement lesion: A systematic review & meta-analysis.Eur J Radiol. 2025 Apr;185:111996. doi: 10.1016/j.ejrad.2025.111996. Epub 2025 Feb 10. Eur J Radiol. 2025. PMID: 39983595
References
-
- Wulfkuhle J.D., Berg D., Wolff C., Langer R., Tran K., Illi J., Espina V., Pierobon M., Deng J., DeMichele A., et al. Molecular Analysis of Her2 Signaling in Human Breast Cancer by Functional Protein Pathway Activation Mapping. Clin. Cancer Res. 2012;18:6426–6435. doi: 10.1158/1078-0432.CCR-12-0452. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

