Multilayer Core-Sheath Structured Nickel Wire/Copper Oxide/Cobalt Oxide Composite for Highly Sensitive Non-Enzymatic Glucose Sensor
- PMID: 40137584
- PMCID: PMC11946131
- DOI: 10.3390/nano15060411
Multilayer Core-Sheath Structured Nickel Wire/Copper Oxide/Cobalt Oxide Composite for Highly Sensitive Non-Enzymatic Glucose Sensor
Abstract
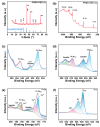
The development of micro glucose sensors plays a vital role in the management and monitoring of diabetes, facilitating real-time tracking of blood glucose levels. In this paper, we developed a three-layer core-sheath microwire (NW@CuO@Co3O4) with nickel wire as the core and copper oxide and cobalt oxide nanowires as the sheath. The unique core-sheath structure of microwire enables it to have both good conductivity and excellent electrochemical catalytic activity when used as an electrode for glucose detecting. The non-enzymatic glucose sensor base on a NW@CuO@Co3O4 core-sheath wire exhibits a high sensitivity of 4053.1 μA mM-1 cm-2, a low detection limit 0.89 μM, and a short response time of less than 2 s.
Keywords: cobalt oxide nanowires; copper oxide; core-sheath; nickel wire; non-enzymatic glucose sensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pohanka M. Glucose Electrochemical Biosensors: The Past and Current Trends. Int. J. Electrochem. Sc. 2021;16:210719. doi: 10.20964/2021.07.52. - DOI
-
- Jarnda K.V., Wang D.Q., Qurrat-Ul-Ain, Anaman R., Johnson V.E., Roberts G.P., Johnson P.S.., Jallawide B.J.R., Kai T.H., Ding P. Recent Advances in Electrochemical Non-Enzymatic Glucose Sensor for the Detection of Glucose in Tears and Saliva: A Review. Sensor Actuat A-Phys. 2023;363:114778. doi: 10.1016/j.sna.2023.114778. - DOI
-
- Zohaa, Arif D., Hassan M., Abdullah M., Miran W., Nasir M.A., Batool S., Baig M.A., Liaqat U. An Electrochemical Sensor Based on Copper Oxide Nanoparticles Loaded on a Mesoporous Mcm-41 for Non-Enzymatic Detection of Glucose. Ceram. Int. 2024;50:12614–12620. doi: 10.1016/j.ceramint.2024.01.171. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

