Folic Acid-Conjugated Magnetic Oleoyl-Chitosan Nanoparticles for Controlled Release of Doxorubicin in Cancer Therapy
- PMID: 40137588
- PMCID: PMC11944324
- DOI: 10.3390/nano15060415
Folic Acid-Conjugated Magnetic Oleoyl-Chitosan Nanoparticles for Controlled Release of Doxorubicin in Cancer Therapy
Abstract
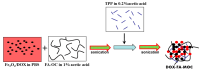
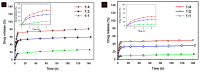
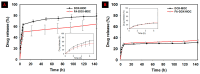
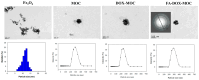
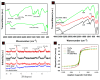
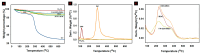
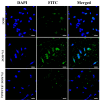
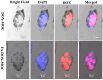
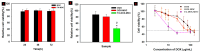
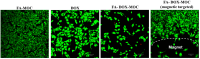
To develop an efficient drug delivery system, we co-entrapped superparamagnetic Fe3O4 and the chemotherapeutic drug doxorubicin (DOX) in oleoyl-chitosan (OC) to prepare DOX-entrapped magnetic OC (DOX-MOC) nanoparticles (NPs) through ionic gelation of OC with sodium tripolyphosphate (TPP). The NPs provide magnetically targeted delivery of DOX in cancer therapy. Using folic acid (FA)-grafted OC, FA-conjugated DOX-entrapped magnetic OC (FA-DOX-MOC) NPs were prepared similarly for FA-mediated active targeting of cancer cells with overexpressed folate receptors. Considering DOX loading and release, the best conditions for preparing DOX-MOC NPs were an OC:TPP mass ratio = 1:4 and OC concentration = 0.2%. These spherical NPs had a particle size of ~250 nm, 87.9% Fe3O4 content, 53.1 emu/g saturation magnetization, 83.1% drug encapsulation efficacy, and 2.81% drug loading efficiency. FA did not significantly change the physico-chemical characteristics of FA-DOX-MOC compared to DOX-MOC, and both NPs showed pH-dependent drug release behaviors, with much faster release of DOX at acidic pH values found in endosomes. However, FA could enhance the intracellular uptake of the NPs and DOX accumulation in the nucleus. This active targeting effect led to significantly higher cytotoxicity towards U87 cancer cells. These results suggest that FA-DOX-MOC NPs can efficiently deliver DOX for controlled drug release in cancer therapy.
Keywords: chitosan; doxorubicin; folic acid; iron oxide; magnetic nanoparticles; oleic acid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in study design, collection, analyses, or interpretation of the data, manuscript writing, or decision to publish the results.
Figures

Similar articles
-
Multi-functional core-shell Fe3O4@Au nanoparticles for cancer diagnosis and therapy.Colloids Surf B Biointerfaces. 2019 Feb 1;174:252-259. doi: 10.1016/j.colsurfb.2018.11.004. Epub 2018 Nov 15. Colloids Surf B Biointerfaces. 2019. PMID: 30469046
-
Biocompatible and Stable GO-Coated Fe3O4 Nanocomposite: A Robust Drug Delivery Carrier for Simultaneous Tumor MR Imaging and Targeted Therapy.ACS Biomater Sci Eng. 2018 Jun 11;4(6):2143-2154. doi: 10.1021/acsbiomaterials.8b00029. Epub 2018 May 9. ACS Biomater Sci Eng. 2018. PMID: 33435038
-
Folate-mediated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery.Eur J Pharm Biopharm. 2010 Sep;76(1):10-6. doi: 10.1016/j.ejpb.2010.05.005. Epub 2010 May 22. Eur J Pharm Biopharm. 2010. PMID: 20472060
-
Engineering a folic acid-decorated ultrasmall gemcitabine nanocarrier for breast cancer therapy: Dual targeting of tumor cells and tumor-associated macrophages.Acta Pharm Sin B. 2022 Mar;12(3):1148-1162. doi: 10.1016/j.apsb.2021.09.024. Epub 2021 Sep 30. Acta Pharm Sin B. 2022. PMID: 35530140 Free PMC article. Review.
-
Doxorubicin-loaded poly(ethylene oxide)-trimellitic anhydride chloride-folate superparamagnetic iron oxide nanoparticles.2010 Jul 16 [updated 2010 Sep 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jul 16 [updated 2010 Sep 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20945566 Free Books & Documents. Review.
References
-
- Mistral J., Ve Koon K.T., Fernando Cotica L., Sanguino Dias G., Aparecido Santos I., Alcouffe P., Milhau N., Pin D., Chapet O., Serghei A., et al. Chitosan-Coated Superparamagnetic Fe3O4 Nanoparticles for Magnetic Resonance Imaging, Magnetic Hyperthermia, and Drug Delivery. ACS Appl. Nano Mater. 2024;7:7097–7110. doi: 10.1021/acsanm.3c06118. - DOI
-
- Li Z., Wan W., Bai Z., Peng B., Wang X., Cui L., Liu Z., Lin K., Yang J., Hao J., et al. Construction of pH-responsive nanoplatform from stable magnetic nanoparticles for targeted drug delivery and intracellular imaging. Sens. Actuators B Chem. 2023;375:132869. doi: 10.1016/j.snb.2022.132869. - DOI
-
- Helmy L.A., Abdel-Halim M., Hassan R., Sebak A., Farghali H.A.M., Mansour S., Tammam S.N. The other side to the use of active targeting ligands; the case of folic acid in the targeting of breast cancer. Colloids Surf. B Biointerfaces. 2022;211:112289. doi: 10.1016/j.colsurfb.2021.112289. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

