Short Peptides from Asian Scorpions: Bioactive Molecules with Promising Therapeutic Potential
- PMID: 40137887
- PMCID: PMC11946205
- DOI: 10.3390/toxins17030114
Short Peptides from Asian Scorpions: Bioactive Molecules with Promising Therapeutic Potential
Abstract
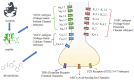
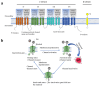
Scorpion venom peptides, particularly those derived from Asian species, have garnered significant attention, offering therapeutic potential in pain management, cancer, anticoagulation, and infectious diseases. This review provides a comprehensive analysis of scorpion venom peptides, focusing on their roles as voltage-gated sodium (Nav), potassium (Kv), and calcium (Cav) channel modulators. It analyzed Nav1.7 inhibition for analgesia, Kv1.3 blockade for anticancer activity, and membrane disruption for antimicrobial effects. While the low targeting specificity and high toxicity of some scorpion venom peptides pose challenges to their clinical application, recent research has made strides in overcoming these limitations. This review summarizes the latest progress in scorpion venom peptide research, discussing their mechanisms of action, therapeutic potential, and challenges in clinical translation. This work aims to provide new insights and directions for the development of novel therapeutic drugs.
Keywords: ion channel blocker; membrane-targeting mechanisms; peptide engineering; scorpion venom peptides; therapeutic potential.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Emerging therapeutic applications of scorpion venom peptides in the Middle East and North Africa: A comprehensive review.Toxicon. 2025 Mar;256:108270. doi: 10.1016/j.toxicon.2025.108270. Epub 2025 Jan 31. Toxicon. 2025. PMID: 39894171 Review.
-
Identification and Characterization of Novel Proteins from Arizona Bark Scorpion Venom That Inhibit Nav1.8, a Voltage-Gated Sodium Channel Regulator of Pain Signaling.Toxins (Basel). 2021 Jul 18;13(7):501. doi: 10.3390/toxins13070501. Toxins (Basel). 2021. PMID: 34357973 Free PMC article.
-
Scorpion Toxin Polyptides as Therapeutic Agents: An Overview.Protein Pept Lett. 2016;23(9):848-59. doi: 10.2174/0929866523666160630184635. Protein Pept Lett. 2016. PMID: 27397476 Review.
-
Discovery of three toxin peptides with Kv1.3 channel and IL-2 cytokine-inhibiting activities from Non-Buthidae scorpions, Chaerilus tricostatus and Chaerilus tryznai.Peptides. 2017 May;91:13-19. doi: 10.1016/j.peptides.2017.03.005. Epub 2017 Mar 12. Peptides. 2017. PMID: 28300672
-
Modulation of hNav by Tst1, a β-toxin purified from the scorpion Tityus stigmurus.Biochimie. 2023 Jan;204:118-126. doi: 10.1016/j.biochi.2022.09.007. Epub 2022 Sep 15. Biochimie. 2023. PMID: 36116743
Cited by
-
Effect of Hottentotta judaicus Scorpion Venom on Nociceptive Response and Inflammatory Cytokines in Mice Using Experimental Hyperalgesia.Molecules. 2025 Jun 26;30(13):2750. doi: 10.3390/molecules30132750. Molecules. 2025. PMID: 40649269 Free PMC article.
References
-
- Cragg G.M., Newman D.J. Natural-Source Medicines Have a Long History in Traditional Medicine. Pure Appl. Chem. 2005;77:1923–1942. doi: 10.1351/pac200577111923. - DOI
-
- Kumar M., Kaur P., Garg R., Patil R.K., Patil H.C. A Study on Antibacterial Property of Curcuma longa—Herbal and Traditional Medicine. Adesh Univ. J. Med. Sci. Res. 2020;2:103–108. doi: 10.25259/AUJMSR_11_2020. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

