Abiotic Degradation of the Toxin Simplexin by Soil Collected from a Pimelea-Infested Paddock
- PMID: 40137897
- PMCID: PMC11946557
- DOI: 10.3390/toxins17030124
Abiotic Degradation of the Toxin Simplexin by Soil Collected from a Pimelea-Infested Paddock
Abstract
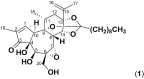
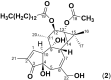
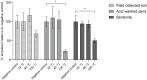
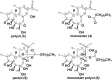
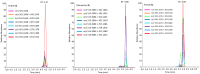
Pimelea poisoning of cattle is caused by the toxin simplexin present in native Pimelea plant species. Surface weathering and burial of Pimelea plant material under soil in Pimelea-infested pastures previously showed simplexin degradation, suggesting soil microbial metabolism and/or abiotic degradation of simplexin in the field. This current study investigated whether soil from a Pimelea-infested paddock was capable of simplexin degradation in the laboratory. The effects of temperature on isolated simplexin levels and simplexin levels in Pimelea plant material treated with field-collected soil, acid-washed sand or bentonite were determined. Pimelea plant material incubated in field-collected soil at 22 °C for seven days did not show any simplexin degradation. Isolated simplexin preadsorbed to field-collected soil, acid-washed sand or bentonite showed simplexin decrease after one hour of incubation at 100 °C with three breakdown products identified by UPLC-MS/MS, indicating that toxin breakdown can be a heat-induced process rather than a microbial-based metabolism. Decreased simplexin levels were observed in Pimelea plant material mixed with acid-washed sand under similar incubation conditions. Overall, the study showed the field-collected soil did not contain soil microorganisms capable of simplexin metabolism within a short period of time. However, the co-exposure to high temperature resulted in significant abiotic simplexin breakdown, without microorganism involvement, with the product structures suggesting that the degradation was a heat promoted acid hydrolysis/elimination process. Overall, this study demonstrated that simplexin breakdown in the field could be a thermal abiotic process with no indication of microbial involvement.
Keywords: Pimelea; hydrolysis; mass spectrometry; plant toxin; simplexin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Fletcher M., Silcock R., Ossedryver S., Milson J., Chow S. Understanding Pimelea Poisoning of Cattle. Department of Employment, Economic Development and Innovation; Brisbane, QLD, Australia: 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

