Safety and efficacy of re-treatment with [177Lu]Lu-DOTA-Octreotate radionuclide therapy in progressive gastro-entero-pancreatic neuroendocrine tumours - a single centre experience
- PMID: 40137978
- PMCID: PMC12316713
- DOI: 10.1007/s00259-025-07235-w
Safety and efficacy of re-treatment with [177Lu]Lu-DOTA-Octreotate radionuclide therapy in progressive gastro-entero-pancreatic neuroendocrine tumours - a single centre experience
Abstract
Aim: Patients with gastro-entero-pancreatic neuroendocrine tumours (GEP NET) who retain somatostatin receptor (SSTR) expression after initial response to [177Lu]Lu-DOTA-Octreotate (LuTate) peptide receptor radionuclide therapy (PRRT) are amenable to re-treatment (R-PRRT) upon progression. We assessed the safety and efficacy of R-PRRT in patients with progressive metastatic GEP NET.
Materials and methods: A retrospective analysis, approved by institutional ethics board, was performed in patients with GEP NET who received R-PRRT for either symptomatically or radiologically progressive disease. Safety was assessed by renal and haematological parameters at 3 months post R-PRRT (CTCAE v5.0). Molecular imaging response was evaluated on [68Ga]Ga-DOTA-Octreotate (GaTate) PET/CT using pre-defined criteria. RECIST 1.1 responses 3 months post R-PRRT were documented when feasible. Progression-free and overall survival analysis were performed.
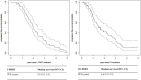
Results: A total of 63 patients had R1-PRRT (1-3 cycles). The majority (70%) had Grade 2 NET and small intestinal primary (51%). A second re-treatment course (R2-PRRT) was given in 20 patients and a third course (R3-PRRT) in 6 patients. Glomerular filtration rate (GFR) was stable following R1-PRRT. Following R2-PRRT, worsening GFR from CTCAE G2 to G3 was seen in 10% (2/20) of patients, but none after R3-PRRT. Grade 3 thrombocytopenia occurred in 2 patients after R1-PRRT and in 1 patient after R3-PRRT. Grade 4 thrombocytopenia was observed in 1 patient post R1-PRRT. Following R1-PRRT, RECIST 1.1 responses CR, PR, SD was 0%, 10%, 76%, respectively. Disease control rate on GaTate PET/CT was 52/58 (89%) post R1-PRRT. Median progression free survival (PFS) following R1-PRRT was 1.6 years (95% CI:1.2-2.3).
Conclusion: R-PRRT is feasible, tolerable and efficacious in achieving disease control in patients with progressive GEP NET.
Keywords: DOTATATERe-Treatment; Gastroenteropancreatic (GEP); Neuroendocrine neoplasms (NENs); PRRTLutetium-177.
© 2025. Crown.
Conflict of interest statement
Declarations. Human ethics and consent to participate declarations: Not applicable. Conflict of interest: No direct conflicts of interests with the current research work.
Figures


References
-
- Fani M, Nicolas GP, Wild D. Somatostatin receptor antagonists for imaging and therapy. J Nucl Med. 2017;58:S61–6. - PubMed
-
- Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–73. - PubMed
-
- Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500–16. - PubMed
-
- Kwekkeboom DJ, Krenning EP. Peptide receptor radionuclide therapy in the treatment of neuroendocrine tumors. Hematol Oncol Clin North Am. 2016;30:179–91. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

