Microglia regulate myelin clearance and cholesterol metabolism after demyelination via interferon regulatory factor 5
- PMID: 40137979
- PMCID: PMC11947375
- DOI: 10.1007/s00018-025-05648-2
Microglia regulate myelin clearance and cholesterol metabolism after demyelination via interferon regulatory factor 5
Abstract
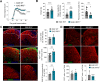
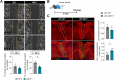
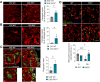
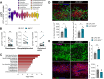
Interferon regulatory factor 5 (IRF5) is a transcription factor that plays a role in orchestrating innate immune responses, particularly in response to viral infections. Notably, IRF5 has been identified as a microglia risk gene linked to multiple sclerosis (MS), but its specific role in MS pathogenesis remains unclear. Through the use of Irf5-/- mice, our study uncovers a non-canonical function of IRF5 in MS recovery. Irf5-/- mice exhibited increased damage in an experimental autoimmune encephalomyelitis (EAE) model and demonstrated impaired oligodendrocyte recruitment into the lesion core following lysolecithin-induced demyelination. Transcriptomic and lipidomic analyses revealed that IRF5 has a role in microglia-mediated myelin phagocytosis, lipid metabolism, and cholesterol homeostasis. Indeed, Irf5-/- microglia phagocytose myelin, but myelin debris is not adequately degraded, leading to an accumulation of lipid droplets, cholesterol esters, and cholesterol crystals within demyelinating lesions. This abnormal buildup can hinder remyelination processes. Importantly, treatments that promote cholesterol transport were found to reduce lipid droplet accumulation and mitigate the exacerbated damage in Irf5-/- mice with EAE. Altogether, our study identified the antiviral transcription factor IRF5 as a key transcriptional regulator of lipid degradation and cholesterol homeostasis and suggest that loss of IRF5 function leads to pathogenic lipid accumulation in microglia, thereby obstructing remyelination. These data and the fact that Irf5 polymorphisms are significantly associated with MS, highlight IRF5 as a potential therapeutic target to promote regenerative responses.
Keywords: Demyelination; IRF5; Lipid homeostasis; Microglia; Multiple sclerosis; Remyelination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors declare that they have no conflicts of interest. Ethical approval: All experiments were performed according to the procedures approved by the Ethics Committee of the University of the Basque Country (UPV/EHU). Animals were handled in accordance with the European Communities Council Directive. The study in human tissue samples was approved by the local human ethics committee (CEISH/18/2010), and all participants provided their written informed consent to take part in the study. The study was performed in accordance with the Declaration of Helsinki.
Figures





References
-
- Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15:545–558. 10.1038/nri3871 - PubMed
-
- Filippi M, Bar-Or A, Piehl F et al (2018) Multiple sclerosis. Nat Rev Dis Prim 4:43. 10.1038/s41572-018-0041-4 - PubMed
-
- Yong VW (2022) Microglia in multiple sclerosis: protectors turn destroyers. Neuron 110:3534–3548. 10.1016/j.neuron.2022.06.023 - PubMed
-
- Distéfano-Gagné F, Bitarafan S, Lacroix S, Gosselin D (2023) Roles and regulation of microglia activity in multiple sclerosis: insights from animal models. Nat Rev Neurosci 24:397–415. 10.1038/s41583-023-00709-6 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

