The PVD neuron has male-specific structure and mating function in Caenorhabditis elegans
- PMID: 40138342
- PMCID: PMC12002248
- DOI: 10.1073/pnas.2421376122
The PVD neuron has male-specific structure and mating function in Caenorhabditis elegans
Abstract
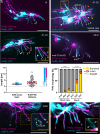
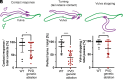
Neurons display unique shapes and establish intricate networks, which may differ between sexes. In complex organisms, studying sex differences in structure and function of individual neurons is difficult. The nematode Caenorhabditis elegans hermaphrodites and males present an exceptional model for studying neuronal morphogenesis in a simple, sexually dimorphic system. We focus on the polymodal sensory bilateral neuron pair PVD, which forms a complex but stereotypic dendritic tree composed of multiple subunits that resemble candelabra. PVD is well studied in hermaphrodites, but not in males. We show here that during larval development, male PVD extends a similar architecture to the hermaphrodite utilizing the sexually shared Menorin patterning mechanism. In early adulthood, however, male PVD develops a unique extension into the copulatory tail structure. Alongside established tail ray neurons RnA and RnB, we show PVD is a third, previously unrecognized, neuron within the tail rays. Unlike RnA and RnB, PVD extends anterogradely, branches and turns within the ray hypodermis, and is nonciliated. This PVD sexually dimorphic arborization is absent in mutant backgrounds which perturb the Menorin guidance complex. SAX-7/L1CAM, a hypodermal component of this complex, shows a male-specific expression pattern which precedes PVD extension, and its presence allows PVD to enter the tail rays. Further, our results reveal that genetically altered arborization or ablation of the PVD results in male mating behavioral defects, particularly as males turn around the hermaphrodite. These results uncover an adult-stage sexual dimorphism of dendritic branching and a function for PVD in male sexual behavior.
Keywords: Caenorhabditis elegans; mating behavior; neuronal arborization; neuronal morphogenesis; sexual dimorphism.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Ghysen A., Dambly-Chaudière C., Aceves E., Jan L.-Y., Jan Y.-N., Sensory neurons and peripheral pathways in Drosophila embryos. Rouxs. Arch. Dev. Biol. 195, 281–289 (1986). - PubMed
-
- White J. G., Southgate E., Thomson J. N., Brenner S., The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340 (1986). - PubMed
-
- Ciofi P., Leroy D., Tramu G., Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 141, 1731–1745 (2006). - PubMed
-
- Jarrell T. A., et al. , The connectome of a decision-making neural network. Science 337, 437–444 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

