Activity-dependent regulation of Cdc42 by Ephexin5 drives synapse growth and stabilization
- PMID: 40138406
- PMCID: PMC11939064
- DOI: 10.1126/sciadv.adp5782
Activity-dependent regulation of Cdc42 by Ephexin5 drives synapse growth and stabilization
Abstract
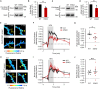
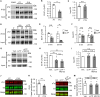
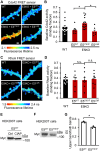
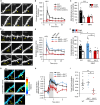
Synaptic Rho guanosine triphosphatase (GTPase) guanine nucleotide exchange factors (RhoGEFs) play vital roles in regulating the activity-dependent neuronal plasticity that is critical for learning. Ephexin5, a RhoGEF implicated in the etiology of Alzheimer's disease and Angelman syndrome, was originally reported in neurons as a RhoA-specific GEF that negatively regulates spine synapse density. Here, we show that Ephexin5 activates both RhoA and Cdc42 in the brain. Furthermore, using live imaging of GTPase biosensors, we demonstrate that Ephexin5 regulates activity-dependent Cdc42, but not RhoA, signaling at single synapses. The selectivity of Ephexin5 for Cdc42 activation is regulated by tyrosine phosphorylation, which is regulated by neuronal activity. Last, in contrast to Ephexin5's role in negatively regulating synapse density, we show that, downstream of neuronal activity, Ephexin5 positively regulates synaptic growth and stabilization. Our results support a model in which plasticity-inducing neuronal activity regulates Ephexin5 tyrosine phosphorylation, driving Ephexin5-mediated activation of Cdc42 and the spine structural growth and stabilization vital for learning.
Figures
References
-
- Holtmaat A., Wilbrecht L., Knott G. W., Welker E., Svoboda K., Experience-dependent and cell-type-specific spine growth in the neocortex. Nature 441, 979–983 (2006). - PubMed
-
- Kasai H., Ziv N. E., Okazaki H., Yagishita S., Toyoizumi T., Spine dynamics in the brain, mental disorders and artificial neural networks. Nat. Rev. Neurosci. 22, 407–422 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

