Retinal gene therapy for Stargardt disease with dual AAV intein vectors is both safe and effective in large animal models
- PMID: 40138422
- PMCID: PMC11939046
- DOI: 10.1126/sciadv.adt9354
Retinal gene therapy for Stargardt disease with dual AAV intein vectors is both safe and effective in large animal models
Abstract
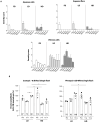
Retinal gene therapy using dual adeno-associated viral (AAV) intein vectors can be applied to genetic forms of blindness caused by mutations in genes with coding sequences that exceed single AAV cargo capacity, such as Stargardt disease (STGD1), the most common inherited macular dystrophy. In view of clinical translation of dual AAV intein vectors, here we set to evaluate both the efficiency and safety of their subretinal administration in relevant large animal models. Accordingly, we have developed the first pig model of STGD1, which we found to accumulate lipofuscin similarly to patients. This accumulation is significantly reduced upon subretinal administration of dual AAV intein vectors whose safety and pharmacodynamics we then tested in nonhuman primates, which showed modest and reversible inflammation as well as high levels of photoreceptor transduction. This bodes well for further clinical translation of dual AAV intein vectors in patients with STGD1 as well as for other blinding diseases that require the delivery of large genes.
Figures



References
-
- Molday R. S., Garces F. A., Scortecci J. F., Molday L. L., Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration. Prog. Retin. Eye Res. 89, 101036 (2022). - PubMed
-
- Cowan C. S., Renner M., De Gennaro M., Gross-Scherf B., Goldblum D., Hou Y., Munz M., Rodrigues T. M., Krol J., Szikra T., Cuttat R., Waldt A., Papasaikas P., Diggelmann R., Patino-Alvarez C. P., Galliker P., Spirig S. E., Pavlinic D., Gerber-Hollbach N., Schuierer S., Srdanovic A., Balogh M., Panero R., Kusnyerik A., Szabo A., Stadler M. B., Orgül S., Picelli S., Hasler P. W., Hierlemann A., Scholl H. P. N., Roma G., Nigsch F., Roska B., Cell types of the human retina and its organoids at single-cell resolution. Cell 182, 1623–1640.e34 (2020). - PMC - PubMed
-
- Lenis T. L., Hu J., Ng S. Y., Jiang Z., Sarfare S., Lloyd M. B., Esposito N. J., Samuel W., Jaworski C., Bok D., Finnemann S. C., Radeke M. J., Redmond T. M., Travis G. H., Radu R. A., Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 115, E11120–E11127 (2018). - PMC - PubMed
-
- Simons E. J., Trapani I., The opportunities and challenges of gene therapy for treatment of inherited forms of vision and hearing loss. Hum. Gene Ther. 34, 808–820 (2023). - PubMed
-
- Tornabene P., Trapani I., Minopoli R., Centrulo M., Lupo M., de Simone S., Tiberi P., Dell’Aquila F., Marrocco E., Iodice C., Iuliano A., Gesualdo C., Rossi S., Giaquinto L., Albert S., Hoyng C. B., Polishchuk E., Cremers F. P. M., Surace E. M., Simonelli F., De Matteis M. A., Polishchuk R., Auricchio A., Intein-mediated protein trans-splicing expands adeno-associated virus transfer capacity in the retina. Sci. Transl. Med. 11, eaav4523 (2019). - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

