Cancer-Associated Fibroblasts Foster a High-Lactate Microenvironment to Drive Perineural Invasion in Pancreatic Cancer
- PMID: 40138590
- PMCID: PMC12167935
- DOI: 10.1158/0008-5472.CAN-24-3173
Cancer-Associated Fibroblasts Foster a High-Lactate Microenvironment to Drive Perineural Invasion in Pancreatic Cancer
Abstract
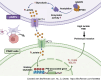
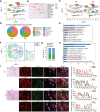
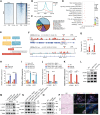
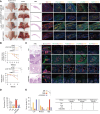
Perineural invasion (PNI) is a pivotal prognostic factor in pancreatic cancer, associated with aggressive tumor behavior and adverse patient outcomes. The recognized clinical impact of PNI highlights the need to better understand the molecular mechanisms underlying PNI-induced phenotypes. In this study, we isolated PNI-associated cancer-associated fibroblasts (pCAF), which demonstrated a markedly enhanced capacity to promote neural invasion in pancreatic cancer compared with non-PNI-associated CAFs. Single-cell, high-throughput sequencing and metabolomics data showed a significant upregulation of glycolysis in pCAFs, fostering a high-lactate tumor microenvironment conducive to cancer progression. pCAF-derived lactate was absorbed by tumor cells, facilitating histone H3K18 lactylation. The lactate-induced epigenetic modification activated the transcription of neural invasion-associated genes, such as L1CAM and SLIT1, thereby driving PNI in pancreatic cancer. Further exploration of metabolic reprogramming in pCAFs revealed enhanced acetylation of the glycolytic enzyme GAPDH, which correlated with increased enzymatic activity and glycolytic flux. Targeting GAPDH and lactylation modifications significantly inhibited neural invasion in a genetically engineered mouse model. Clinical data suggested that high levels of H3K18 lactylation correlate with severe PNI and poorer patient prognosis. Together, these findings provide critical insights into the role of CAFs in promoting PNI of pancreatic cancer, highlighting glycolytic reprogramming and lactate-driven histone modifications as potential therapeutic targets for PDAC.
Significance: Targeting cancer-associated fibroblast metabolism or histone lactylation in pancreatic cancer cells to reverse epigenetic remodeling induced by lactate accumulation in the tumor microenvironment are potential therapeutic strategies to inhibit perineural invasion.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
No disclosures were reported.
Figures




References
-
- Bapat A A, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer 2011;11:695–707. - PubMed
-
- Demir IE, Boldis A, Pfitzinger P L, Teller S, Brunner E, Klose N, et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst 2014;106:dju184. - PubMed
-
- Lin Q, Zheng S, Yu X, Chen M, Zhou Y, Zhou Q, et al. Standard pancreatoduodenectomy versus extended pancreatoduodenectomy with modified retroperitoneal nerve resection in patients with pancreatic head cancer: a multicenter randomized controlled trial. Cancer Commun (Lond) 2023;43:257–75. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

