Methotrexate, Doxorubicin, and Cisplatin Versus Methotrexate, Doxorubicin, and Cisplatin + Ifosfamide in Poor Responders to Preoperative Chemotherapy for Newly Diagnosed High-Grade Osteosarcoma (JCOG0905): A Multicenter, Open-Label, Randomized Trial
- PMID: 40138604
- PMCID: PMC12118622
- DOI: 10.1200/JCO-24-01281
Methotrexate, Doxorubicin, and Cisplatin Versus Methotrexate, Doxorubicin, and Cisplatin + Ifosfamide in Poor Responders to Preoperative Chemotherapy for Newly Diagnosed High-Grade Osteosarcoma (JCOG0905): A Multicenter, Open-Label, Randomized Trial
Abstract
Purpose: Our previous NECO phase II studies on high-grade osteosarcoma suggested that administering ifosfamide (IF; 16 g/m2 [4g/m2 once on day 1, then 2g/m2 once on days 2-7] × six) to patients showing a poor response (PrRsp) to preoperative chemotherapy with methotrexate, doxorubicin, and cisplatin (MAP) improves their prognoses. In this Japan Clinical Oncology Group (JCOG) study, JCOG0905, we aimed to investigate the efficacy and safety of IF in patients with PrRsp.
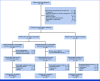
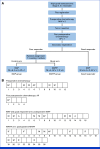
Methods: JCOG0905 is a multicenter, open-label, multi-institutional, randomized trial. Eligible patients (50 years and younger) had resectable, high-grade osteosarcoma (stage II or III, Union for International Cancer Control TNM) of the extremities, limb girdles, and thoracic wall. After two MAP cycles and tumor resection, patients with PrRsp were randomly assigned to either the MAP or MAP plus 15 g/m2 (3g/m2 once daily on days 1-5) × six IF (MAP + IF [MAPIF]) group. The primary end point was disease-free survival (DFS); secondary end points were overall survival (OS) and safety. The planned sample size was 100 patients with a one-sided α of .1 and a power of 0.7, assuming a 3-year DFS of 50% and 65% for MAP and MAPIF, respectively. This trial is registered with the Japan Registry of Clinical Trials (jRCT; jRCTs031180126).
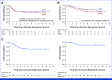
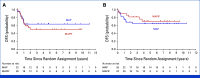
Results: Of the 287 patients registered between February 2010 and August 2020, 51 and 52 patients with PrRsp were assigned to the MAP and MAPIF groups, respectively. As of March 2022, DFS did not differ between groups (hazard ratio [HR], 1.05 [95% CI, 0.55 to 1.98]) and OS was numerically inferior in the MAPIF group (HR, 1.48 [95% CI, 0.68 to 3.22]). Nine and zero patients in the MAPIF and MAP groups discontinued treatment because of adverse events, respectively.
Conclusion: Evidence from JCOG0905 does not support the addition of IF for patients with PrRsp.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Eilber F, Giuliano A, Eckardt J, et al. Adjuvant chemotherapy for osteosarcoma: A randomized prospective trial. J Clin Oncol. 1987;5:21–26. - PubMed
-
- Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. - PubMed
-
- Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. - PubMed
-
- Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. 2015;33:2279–2287. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

