Targeted translation inhibition of chloroplast and mitochondrial mRNAs by designer pentatricopeptide repeat proteins
- PMID: 40138717
- PMCID: PMC11941472
- DOI: 10.1093/nar/gkaf222
Targeted translation inhibition of chloroplast and mitochondrial mRNAs by designer pentatricopeptide repeat proteins
Abstract
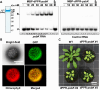
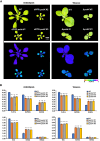
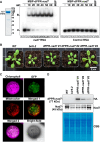
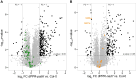
Pentatricopeptide repeat (PPR) proteins are crucial for organellar gene expression. To establish a tool for gene expression manipulation in Arabidopsis plastids and genetically inaccessible mitochondria, we engineered designer (dPPR) proteins to specifically inhibit the translation of organellar mRNAs by masking their start codons. Unlike prior methods for targeted downregulation of gene expression, which rely on re-targeting native PPR proteins to RNA sequences closely related to their original targets, our approach employs a synthetic P-type PPR scaffold that can be designed to bind any RNA sequence of interest. Here, using dPPR-psbK and dPPR-nad7, we targeted the psbK mRNA in chloroplasts and the nad7 mRNA in mitochondria, respectively. dPPR-psbK effectively bound to psbK mRNA and inhibited its translation with high specificity, resulting in disrupted PSII supercomplexes and reduced photosynthetic efficiency. dPPR-nad7 suppressed nad7 translation, affecting NADH oxidase activity in complex I and growth retardation. Comparing phenotypes with tobacco psbK knockouts and nad7 knockdown bir6-2 mutants, along with quantitative proteomics, showed no clear evidence of physiologically relevant off-target effects. Our findings establish dPPR proteins as precise tools for targeted translation inhibition, facilitating functional studies of organellar genes and offering a novel approach with potential for manipulating organellar gene expression in diverse plant species.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

