ACVR2A attenuation impacts lactate production and hyperglycolytic conditions attracting regulatory T cells in hepatocellular carcinoma
- PMID: 40139191
- PMCID: PMC12047472
- DOI: 10.1016/j.xcrm.2025.102038
ACVR2A attenuation impacts lactate production and hyperglycolytic conditions attracting regulatory T cells in hepatocellular carcinoma
Abstract
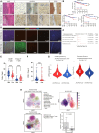
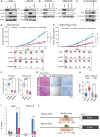
Although ACVR2A mutations are prevalent in non-viral hepatocellular carcinomas (HCCs), the underlying mechanism remains unelucidated. Our molecular investigation reveals that ACVR2A impairment induces hyperglycolysis through the inactivation of the SMAD signaling pathway. Using syngeneic transplantation models and human clinical samples, we clarify that ACVR2A-deficient HCC cells produce and secrete lactate via the upregulation of lactate dehydrogenase A (LDHA) and monocarboxylate transporter 4 (MCT4) expression levels, which promotes regulatory T (Treg) cell accumulation and then acquires resistance to immune checkpoint inhibitors. Remarkably, genetic knockdown and pharmacological inhibition of MCT4 ameliorate the high-lactate milieu in ACVR2A-deficient HCC, resulting in the suppression of intratumoral Treg cell recruitment and the restoration of the sensitivity to PD-1 blockade. These findings furnish compelling evidence that lactate attenuates anti-tumor immunity and that therapeutics targeting this pathway present a promising strategy for mitigating immunotherapy resistance in ACVR2A-deficient HCC.
Keywords: ACVR2A; LDHA; MCT4; PD-1; hepatocellular carcinoma; immunotherapy; lactate; regulatory T cell.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
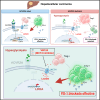
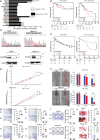
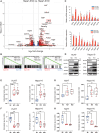
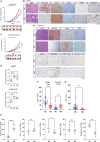


References
-
- Nahon P., Bamba-Funck J., Layese R., Trépo E., Zucman-Rossi J., Cagnot C., Ganne-Carrié N., Chaffaut C., Guyot E., Ziol M., et al. Integrating genetic variants into clinical models for hepatocellular carcinoma risk stratification in cirrhosis. J. Hepatol. 2023;78:584–595. doi: 10.1016/j.jhep.2022.11.003. - DOI - PubMed
-
- Trépo E., Caruso S., Yang J., Imbeaud S., Couchy G., Bayard Q., Letouzé E., Ganne-Carrié N., Moreno C., Oussalah A., et al. Common genetic variation in alcohol-related hepatocellular carcinoma: a case-control genome-wide association study. Lancet Oncol. 2022;23:161–171. doi: 10.1016/s1470-2045(21)00603-3. - DOI - PubMed
-
- Villanueva A., Alsinet C., Yanger K., Hoshida Y., Zong Y., Toffanin S., Rodriguez-Carunchio L., Solé M., Thung S., Stanger B.Z., Llovet J.M. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660–1669.e7. doi: 10.1053/j.gastro.2012.09.002. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

