A once-daily GLP-1/GIP/glucagon receptor tri-agonist (NN1706) lowers body weight in rodents, monkeys and humans
- PMID: 40139439
- PMCID: PMC12051155
- DOI: 10.1016/j.molmet.2025.102129
A once-daily GLP-1/GIP/glucagon receptor tri-agonist (NN1706) lowers body weight in rodents, monkeys and humans
Abstract
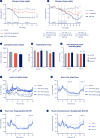
Single molecules that combine complementary modes of action with glucagon-like peptide-1 receptor (GLP-1R) agonism are best-in-class therapeutics for obesity treatment. NN1706 (MAR423, RO6883746) is a fatty-acylated tri-agonist designed for balanced activity at GLP-1R and glucose-dependent insulinotropic peptide receptor (GIPR) with lower relative potency at the glucagon receptor (GcgR). Obese mice, rats and non-human primates dosed with NN1706 showed significant body weight reductions and improved glycemic control. In human participants with overweight or obesity, daily subcutaneous NN1706 treatment resulted in substantial body weight loss in a dose-dependent manner without impairing glycemic control (NCT03095807, NCT03661879). However, increased heart rate was observed across NN1706 treatment cohorts, which challenges further clinical development of NN1706.
Keywords: Clinical; Glucagon; Incretins; Obesity.
Copyright © 2025 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest Brian Finan: Shareholder and employee of Eli Lilly; shareholder and former employee of Novo Nordisk A/S Jonathan D. Douros: Shareholder and former employee of Novo Nordisk A/S Ronald Goldwater: Employee of Parexel International Ann Maria Kruse Hansen: Shareholder and employee of Novo Nordisk A/S Julie B. Hjerpsted: Shareholder and employee of Novo Nordisk A/S Karina Rahr Hjøllund: Shareholder and employee of Novo Nordisk A/S Martin K. Kankam: Employee at Altasciences Clinical Kansas, Inc., which has received research funding from Novo Nordisk A/S, Merck, Vertex, Camino, Ionis, EncuraGen Inc., Eliem Therapeutics, Arthrosi Therapeutic, Staidson Biopharma, NIAID/NIH, Amgen and Biogen Patrick J. Knerr: Shareholder and former employee of Novo Nordisk A/S Anish Konkar: Shareholder and employee of AstraZeneca Stephanie A. Mowery: Shareholder and former employee of Novo Nordisk A/S Timo D. Müller: receives research funding from Novo Nordisk and has received speaking fees from Eli Lilly, AstraZeneca, Novo Nordisk and Merck John Rømer Nielsen: Shareholder and employee Novo Nordisk A/S Sune Boris Nygård: Shareholder and employee Novo Nordisk A/S Diego Perez-Tilve: Received research funds from Novo Nordisk A/S Kirsten Raun: Shareholder and employee of Novo Nordisk A/S Bin Yang: Employee of Dexatide LLC; shareholder and former employee of Novo Nordisk A/S Matthias H. Tschöp: Advisory board of ERX Pharmaceuticals, Inc., Cambridge, MA (2019), Research Cluster Advisory Panel (ReCAP) of the Novo Nordisk Foundation (2017–2019), research funding from Novo Nordisk (2016–2020) and Sanofi-Aventis (2012–2019), consultations for Böhringer Ingelheim Pharma GmbH & Co. KG (2020 & 2021), scientific lectures for Sanofi-Aventis Deutschland GmbH (2020) and Astra-Zeneca GmbH (2024); As CEO and CSO of Helmholtz Munich, co-responsible for collaborations of the employees with a multitude of companies and institutions worldwide, including but not limited to Boehringer Ingelheim, Novo Nordisk A/S, Roche Diagnostics, Arbormed, Eli Lilly, SCG Cell Therapy and others, and overall responsible for commercial technology transfer activities. Richard D. DiMarchi: Shareholder and former employee of Novo Nordisk A/S; Co-inventor of intellectual property at Indiana University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Sumithran P., Prendergast L.A., Delbridge E., Purcell K., Shulkes A., Kriketos A., et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. - PubMed
-
- Novo Nordisk . 2021. WEGOVY (semaglutide) injection, for subcutaneous use.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215256s000lbl.pdf
-
- Novo Nordisk . 2018. SAXENDA (liraglutide) injection, for subcutaneous use.https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206321s007lbl.pdf
-
- Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

