TRanscutaneous lImb reCovEry Post-Stroke (TRICEPS): study protocol for a randomised, controlled, multiarm, multistage adaptive design trial
- PMID: 40139703
- PMCID: PMC11950934
- DOI: 10.1136/bmjopen-2024-092520
TRanscutaneous lImb reCovEry Post-Stroke (TRICEPS): study protocol for a randomised, controlled, multiarm, multistage adaptive design trial
Abstract
Introduction: Arm weakness after stroke is one of the leading causes of adult-onset disability. Invasive vagus nerve stimulation (VNS) paired with rehabilitation has been shown to improve arm recovery in chronic stroke. Small studies of non-invasive or transcutaneous VNS (tVNS) suggest it is safe and tolerable. However, it is not known whether tVNS paired with rehabilitation is effective in promoting arm recovery in chronic stroke and what the mechanisms of action are.
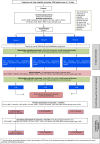
Methods and analysis: TRICEPS is a UK multicentre, double-blinded, superiority, parallel-group, three-arm two-stage with an option to select promising arm(s) at 50% accrual, individually randomised, sham-controlled trial. Up to 243 participants will be randomised (1:1:1) using minimisation via a restricted, web-based centralised system. tVNS will be delivered by a movement-activated tVNS system (TVNS Technologies), which delivers stimulation during repetitive task practice. Rehabilitation will consist of repetitive task training for 1 hour a day, 5 days per week for 12 weeks. Participants will be adults with anterior circulation ischaemic stroke between 6 months and 10 years prior with moderate-severe arm weakness. The primary outcome measure will be the change in Upper Limb Fugl-Meyer total motor score at 91 days after the start of treatment. Secondary outcome measures include the Wolf Motor Function Test, the Modified Ashworth Scale to assess spasticity in the affected arm and the Stroke-Specific Quality of Life Scale. A mechanistic substudy including 40 participants will explore the mechanisms of active versus sham tVNS using multimodal MRI and serum inflammatory cytokine levels. Participant recruitment started on 30 November 2023.
Ethics and dissemination: The study has received ethical approval from the Cambridge Central Research Ethics Committee (REC reference: 22/NI/0134). Dissemination of results will be via publications in scientific journals, meetings, written reports and articles in stakeholder publications.
Trial registration number: ISRCTN20221867.
Keywords: Clinical trials; Rehabilitation medicine; Stroke.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–6. doi: 10.1161/01.STR.0000087172.16305.CD. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
