Ozoralizumab shows effectiveness regardless of baseline RF and ACPA titres in patients with RA: a post hoc analysis of the OHZORA trial
- PMID: 40139716
- PMCID: PMC12212905
- DOI: 10.1093/rheumatology/keaf171
Ozoralizumab shows effectiveness regardless of baseline RF and ACPA titres in patients with RA: a post hoc analysis of the OHZORA trial
Abstract
Objectives: Ozoralizumab (OZR) is a next-generation anti-TNF NANOBODY® compound. The primary objective was to evaluate the efficacy of OZR in patients with RA in varying RF and anti-citrullinated peptide antibody (ACPA) titres. The secondary objective was to evaluate the changes in RF and ACPA titres following OZR treatment.
Methods: A post hoc analysis was conducted on data from the Phase II/III OHZORA trial, which included 381 Japanese patients with RA who were treated with either 30 or 80 mg OZR over 52 weeks after demonstrating an inadequate response to MTX. Patients were classified into four groups based on the baseline RF or ACPA titre quartiles. The disease activity scores, RF and ACPA titres, plasma OZR concentrations and OZR-neutralizing antibody levels were evaluated. Statistical analyses included the Kruskal-Wallis test, two-way ANOVA and correlation analysis.
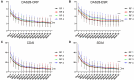
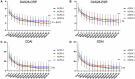
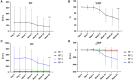
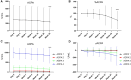
Results: Treatment with OZR 30 mg significantly reduced disease activity in all the groups (P < 0.001), and the reduction in disease activity scores was comparable among the groups. Treatment with OZR 30 mg decreased the mean RF and ACPA titres from 149.5 to 71.1 IU/ml (P < 0.001) and 299.6 to 237.6 U/ml (P < 0.001), respectively. Effective trough concentrations of OZR were maintained for up to 52 weeks in all the groups, and baseline RF and ACPA titres were not associated with the generation of OZR-neutralizing antibodies.
Conclusion: OZR is an effective anti-TNF treatment for RA, regardless of RF and ACPA titres, and exhibits promise as a novel therapeutic option.
Keywords: NANOBODY®; RA; RF; VHH antibody; inhibitor; ozoralizumab; tumour necrosis factor-α.
© The Author(s) 2025. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Feldmann M, Maini RN, Soriano ER, Strand V, Takeuchi T. 25 years of biologic DMARDs in rheumatology. Nat Rev Rheumatol 2023;19:761–6. - PubMed
-
- Keam SJ. Ozoralizumab: first approval. Drugs 2023;83:87–92. - PubMed
-
- Takeuchi T. Structural, nonclinical, and clinical features of ozoralizumab: a novel tumour necrosis factor inhibitor. Mod Rheumatol 2023;33:1059–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

