Oxaliplatin, ATR inhibitor and anti-PD-1 antibody combination therapy controls colon carcinoma growth, induces local and systemic changes in the immune compartment, and protects against tumor rechallenge in mice
- PMID: 40139833
- PMCID: PMC11950992
- DOI: 10.1136/jitc-2024-010791
Oxaliplatin, ATR inhibitor and anti-PD-1 antibody combination therapy controls colon carcinoma growth, induces local and systemic changes in the immune compartment, and protects against tumor rechallenge in mice
Abstract
Background: Colorectal cancer (CRC) is the third most common cancer type and one of the leading causes of cancer-related death worldwide. The treatment of advanced metastatic CRC relies on classical chemotherapy combinations (5-fluorouracil, oxaliplatin or irinotecan). However, their use is limited by the emergence of resistance mechanisms, including to oxaliplatin. In this context, we recently showed that the combination of oxaliplatin and ataxia telangiectasia and Rad3-related protein inhibition (VE-822) is synergistic and may have a potential therapeutic effect in metastatic CRC management.
Methods: In this study, we investigated the role of the VE-822+oxaliplatin (Vox) combination on the immune response and its potential synergy with an anti-programmed-cell Death receptor-1 (PD-1) antibody. We used cell lines and organoids from metastatic CRC to investigate in vitro Vox efficacy and orthotopic syngeneic mouse models of metastatic CRC to assess the efficacy of Vox+anti-PD-1 antibody and identify the involved immune cells.
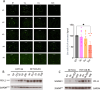
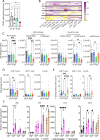
Results: The Vox+anti-PD-1 antibody combination completely cured tumor-bearing mice and protected them from a rechallenge. Vox was associated with a reduction of tumor-infiltrated neutrophils, CD206+ macrophages and regulatory T cells. Vox also induced a deep depletion of blood neutrophils. The increased bone marrow granulopoiesis failed to compensate for the Vox-mediated mature neutrophil depletion. Neutrophil depletion using a mouse recombinant anti-Ly6G antibody partially mimicked the Vox effect on the tumor microenvironment, but to a lower extent compared with the Vox+anti-PD-1 antibody combination. Vox, but not neutrophil depletion, led to the emergence of an Ly6C+ PD-1+ CD8+ T-cell population in the blood and spleen of tumor-harboring mice. These cells were proliferating, and expressed IFN-γ, CD62L, CXCR3 and Eomes. Moreover, the proportion of tumor antigen-specific T cells and of CD122+ BCL6+ T cells, which shared phenotypic characteristics with stem-like CD8+ T cells, was increased in treated mice.
Conclusions: Our work strongly suggests that the Vox+anti-PD-1 antibody combination might significantly improve survival in patients with metastatic and treatment-refractory CRC by acting both on cancer cells and CD8+ T cells.
Keywords: Colorectal Cancer; Immune Checkpoint Inhibitor; Neutropenia; T cell.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures





References
-
- Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709–19. doi: 10.1016/S1470-2045(16)30500-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
