Metabolic and Pharmacokinetic Profiling of a Ketone Ester by Background SGLT2 Inhibitor Therapy in HFrEF
- PMID: 40139871
- PMCID: PMC12013845
- DOI: 10.1016/j.jacbts.2024.10.014
Metabolic and Pharmacokinetic Profiling of a Ketone Ester by Background SGLT2 Inhibitor Therapy in HFrEF
Abstract
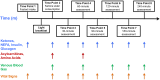
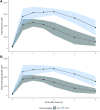
Growing evidence supports therapeutic ketosis in heart failure with reduced ejection fraction, though uncertainty exists regarding use with SGLT2i and dose-dependent effects. In a phase I trial of 2 ketone ester (KE) doses in 20 heart failure with reduced ejection fraction participants, stratified by background SGLT2i, the authors detailed pharmacokinetic parameters, noting rapid ketosis and short half-life. KE was associated with lower non-esterified fatty acid, branched-chain amino acids, and most acylcarnitines (except C2 and C4-OH, which increased); differences were observed by SGLT2i and KE dose. Increases in heart rate and decreases in systolic blood pressure, pH, and bicarbonate were generally transient. KE ingestion induces rapid changes in key metabolic pathways, differentially affected by SGLT2i (fatty acid metabolism) and KE dose (ketone metabolism). Hemodynamic effects were transient and irrespective of dose or SGLT2i. (Ketone Pharmacokinetic Study in HFrEF; NCT05757193).
Keywords: SGLT2 inhibitor; acylcarnitine; heart failure with reduced ejection fraction; insulin; ketone bodies; metabolomics.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This trial was funded by the National Heart, Lung, and Blood Institute grant K23HL161348 (Dr Selvaraj) and the American Heart Association grant #935275 (Dr Selvaraj). The metabolomics core laboratory was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant P30DK124723 (Dr Newgard). Dr Kelly is supported by National Institutes of Health (NIH) grant R01 HL151345. Dr Zamani is supported by NIH grants R01 HL155599, R01 HL157264, R01 HL149722, U01-HL160277, and UH3DK128298. Dr Selvaraj receives research support from the National Heart, Lung, and Blood Institute (grant K23HL161348), American Heart Association (grant #935275), Doris Duke Charitable Foundation (grant #2020061), the Mandel Foundation, Duke Heart Center Leadership Council, the Institute for Translational Medicine and Therapeutics, and Foundation for Sarcoidosis Research; and has participated in advisory boards for AstraZeneca. Dr Thompson receives support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (grant T32HD104576) and the National Institute of General Medicine Sciences of the National Institutes of Health (grant T32GM086330). Dr Hornik has received support from the NHLBI grant 1K24HL173596 and is a consultant for Cytokinetics Inc. Dr Mentz received research support and honoraria from Abbott, Alleviant Medical, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Eli Lilly, Lexicon, Medtronic, Medable, Merck, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Relypsa, Reprieve Cardiovascular, Respicardia, Roche, Rocket Pharmaceuticals, Sanofi, Verily, Vifor, Windtree Therapeutics, and Zoll. Dr Cade is a consultant for Stealth Biotherapeutics and Pharmanovia UK. Dr Kelly is a consultant for Pfizer Ltd. and Amgen. Dr Zamani receives research support from Amgen and has consulted for Pfizer and Vyaire. Dr Shah receives research funding through a sponsored research agreement to Duke University from Astra Zeneca, Lilly Inc., Verily Inc. and nference; she is a coinventor on unlicensed patents held by Duke University. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

