Homeostatic Role of Decorin in Right Ventricular Pressure Overload and Pulmonary Hypertension Induced Remodeling
- PMID: 40139874
- PMCID: PMC12013849
- DOI: 10.1016/j.jacbts.2024.10.007
Homeostatic Role of Decorin in Right Ventricular Pressure Overload and Pulmonary Hypertension Induced Remodeling
Abstract
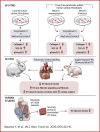
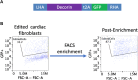
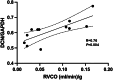
Right ventricular (RV) pressure loading induces RV profibrotic signaling and fibrosis associated with RV dysfunction. RV decorin protein levels are decreased in patients with chronic RV pressure loading. RV decorin protein levels are also decreased in 4 animal models of mechanical RV pressure loading and pulmonary arterial hypertension. Human cardiac fibroblasts overexpressing decorin show diminished collagen-1 secretion in response to mechanical or chemical profibrotic stress while decorin knockout human cardiac fibroblasts show increased collagen-1 secretion in response to stress. Downregulation of decorin may play a key role in upregulating transforming growth factor-β1 profibrotic signaling and fibrosis that contribute to RV dysfunction in RV pressure loading.
Keywords: cardiac fibrosis; decorin; pulmonary hypertension; right ventricular dysfunction; right ventricular pressure loading; transforming growth factor-β1.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported in part by the Heart and Stroke Foundation of Canada and by the Canadian Institutes of Health Research (FRN 16226). Dr Connelly holds the Keenan Chair for Research Leadership, Keenan Research Centre for Biomedical Science, Toronto. All other authors have reported they have no relationships relevant to the contents of this paper to disclose.
Figures






References
-
- van der Bruggen C.E.E., Tedford R.J., Handoko M.L., van der Velden J., de Man F.S. RV pressure overload: from hypertrophy to failure. Cardiovasc Res. 2017;113:1423–1432. - PubMed
-
- Andersen S., Nielsen-Kudsk J.E., Vonk Noordegraaf A., de Man F.S. Right ventricular fibrosis. Circulation. 2019;139:269–285. - PubMed
-
- Babu-Narayan S.V., Kilner P.J., Li W., et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–413. - PubMed
-
- Yamamura K., Yuen D., Hickey E.J., et al. Right ventricular fibrosis is associated with cardiac remodeling after pulmonary valve replacement. Heart. 2019;105:855–863. - PubMed
LinkOut - more resources
Full Text Sources

