Spermidine Enhances Mitochondrial Function and Mitigates Aortic Valve Calcification: Implications for DNA Methyltransferase-1 Activity
- PMID: 40139876
- PMCID: PMC12013848
- DOI: 10.1016/j.jacbts.2024.11.011
Spermidine Enhances Mitochondrial Function and Mitigates Aortic Valve Calcification: Implications for DNA Methyltransferase-1 Activity
Abstract
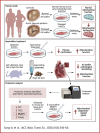
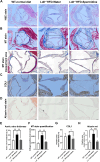
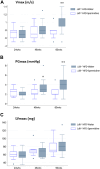
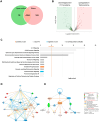
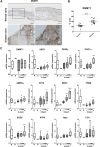
Aortic stenosis (AS) is a severe heart valve disease marked by calcification, leading to heart failure. This study examined mitochondrial function in human aortic valve interstitial cells isolated from patients with AS and tested spermidine, an autophagy inducer as AS treatment. Spermidine treatment reduced fibrosis and calcification in human aortic valve interstitial cells and improved these features in spermidine-treated mice. The AKT-TP53-DNMT1-PPARG pathway was implicated, and DNA methyltransferase 1 inhibition by 5-azacytidine enhanced mitochondrial biogenesis by reducing mitochondrial DNA hypermethylation. These findings suggest that spermidine or DNA methyltransferase 1 inhibition could prevent aortic valve disease by improving mitochondrial function.
Keywords: DNA methyltransferase 1; aging; aortic stenosis; mitochondrial function; spermidine.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2019R1I1A2A01060702) and the Korean government (MSIT) (RS-2024-00345819). It was also supported by a grant (2022IF-0004) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea. Dr Aikawa’s lab is supported by National Institute of Health grants R01 HL147095 and R01 HL141917. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
-
- Boskovski M.T., Gleason T.G. Current therapeutic options in aortic stenosis. Circ Res. 2021;128:1398–1417. - PubMed
-
- Eveborn G.W., Schirmer H., Heggelund G., Lunde P., Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø Study. Heart. 2013;99:396–400. - PubMed
-
- Joseph J., Naqvi S.Y., Giri J., Goldberg S. Aortic stenosis: pathophysiology, diagnosis, and therapy. Am J Med. 2017;130:253–263. - PubMed
-
- Kanwar A., Thaden J.J., Nkomo V.T. Management of patients with aortic valve stenosis. Mayo Clin Proc. 2018;93:488–508. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

