Invasive vs Conservative Management of Patients With Chronic Total Occlusion: Results From the ISCHEMIA Trial
- PMID: 40139890
- PMCID: PMC12284807
- DOI: 10.1016/j.jacc.2025.01.029
Invasive vs Conservative Management of Patients With Chronic Total Occlusion: Results From the ISCHEMIA Trial
Abstract
Background: Randomized trials of chronic total occlusion (CTO) revascularization vs medical therapy have yielded inconsistent results.
Objectives: The aim of this study was to evaluate outcomes with an initial invasive strategy (INV) vs an initial conservative strategy (CON) in patients with coronary computed tomographic angiography (CCTA)-determined CTO in the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial.
Methods: Participants in ISCHEMIA who underwent CCTA evaluated for CTO by the core laboratory (3,113 of 5,179 randomized patients [60%]) were categorized into subgroups with (100% stenosis) and without (<100% stenosis) CTO. Primary analysis compared outcomes in those randomized to INV vs CON using an intention-to-treat approach. Secondary analyses compared outcomes using inverse probability weighting to model successful CTO revascularization (REV) in all INV participants vs CON participants.
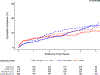
Results: Of the 3,113 CCTA-evaluable participants, 1,470 had at least 1 CTO (752 INV and 718 CON). INV did not reduce cardiovascular (CV) death or myocardial infarction (MI) (5-year difference -3.5%; 95% CI: -7.8% to 0.8%) and resulted in more procedural MIs (2.5%; 95% CI: 1.0%-4.0%) but fewer spontaneous MIs (-6.3%; 95% CI: -9.7% to -3.2%) than CON. CTO REV modeled across INV had a high probability (>90%) of any lower CV death or MI, MI, spontaneous MI, unstable angina, and heart failure counterbalanced by a higher rate of procedural MI. CTO REV significantly improved angina-related quality of life (mean difference 4.6 points), Rose Dyspnea Scale score (rescaled) (mean difference 5.3 points), and EQ-5D visual analog scale score (4.6 points).
Conclusions: In the ISCHEMIA trial, the risks and benefits of INV compared with CON were similar among patients with and without CCTA-determined CTO (more frequent procedural MI, less frequent spontaneous MI, and significantly improved angina and dyspnea-related quality of life). In an observational comparison, successful CTO REV was associated with a high probability of lower CV death or MI (driven by lower MI) compared with CON. (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches [ISCHEMIA]; NCT01471522).
Keywords: chronic total occlusion; percutaneous coronary intervention; revascularization; stent; surgery.
Copyright © 2025 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This research was supported by National Institutes of Health grants U01HL105907, U01HL105462, U01HL105561, U01HL105565, and T32HL079896. This project was supported by the National Heart, Lung, and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Institutes of Health, or the U.S. Department of Health and Human Services. Dr Bangalore received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; has received grants and personal fees from Abbott Vascular; and has received personal fees from Biotronik, Pfizer, Amgen, and Reata (outside the submitted work). Dr Mancini has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; has received grants and personal fees from Amgen, Sanofi, Boehringer Ingelheim, AstraZeneca, Bayer, Janssen, Novo Nordisk, Novartis, and HLS Therapeutics (outside the submitted work). Dr Leipsic is a consultant for and has stock options with HeartFlow and Circle Cardiovascular Imaging; and has received a research grant from GE Healthcare (outside the submitted work). Dr Budoff has received grant support from GE (outside the submitted work). Dr Xu has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Anthopolos has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Brilakis has received consulting and speaker honoraria from Abbott Vascular, the American Heart Association (associate editor, Circulation), Biotronik, Boston Scientific, the Cardiovascular Innovations Foundation (board of directors), Cardiovascular Systems, Elsevier, GE Healthcare, IMDS, Medtronic, and Teleflex; has received research support from Boston Scientific and GE Healthcare; is an owner of Hippocrates; and is a shareholder in MHI Ventures, Cleerly Health, and Stallion Medical. Dr Spertus has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; has provided consultative services on patient-reported outcomes and evidence evaluation to Abbott, Alnylam, AstraZeneca, Bayer, Janssen, Bristol Myers Squibb, Edwards Lifesciences, Terumo, Cytokinetics, and Imbria; has received research grants from Bristol Myers Squibb, Cytokinetics, Imbria, and Janssen; owns the copyright to the SAQ-7, the Kansas City Cardiomyopathy Questionnaire, and the Peripheral Artery Questionnaire; and serves on the board of directors for Blue Cross and Blue Shield of Kansas City. Dr Mark received grants from the National Institutes of Health and the American Heart Association during the conduct of the study; has received grants from HeartFlow and Novo Nordisk (outside the scope of the submitted work); and has received consulting fees from Boehringer Ingelheim, Novartis, and CeleCor (outside the scope of the submitted work). Dr Hague has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; and has received consultant and speaker fees from VIDA Diagnostics and Boehringer Ingelheim. Dr Min has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; is an employee of and holds equity in Cleerly; has served on the scientific advisory board for Arineta and Tourmaline Bio; and has received grants and other from GE Healthcare (outside the submitted work). Dr Reynolds has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; has received nonfinancial support from Abbott Vascular, Siemens, and BioTelemetry (outside the submitted work). Dr Elghamaz received grants from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Nair has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Mavromatis has received grants from the National Heart, Lung, and Blood Institute (CV Inflammation Reduction Trial and GMCSF in PAD-3 trial), CSL Behring, St Jude Medical, Medtronic, DalCor Pharmaceuticals, AstraZeneca, Novartis, and Regeneron; and is a member of the American College of Cardiology and the Society for Cardiovascular Angiography and Interventions. Dr Gosselin received grants from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Subhash has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; has received consulting honoraria from Medtronic, AstraZeneca, and Livmor; and has received institutional research grants from Boston Scientific and Chiesi. Dr Grantham has received institutional research grants, speaker fees, and honoraria from Boston Scientific and Asahi Intecc; and has received advisory board fees from Teleflex and Boston Scientific. Dr Williams has received grants from the National Heart, Lung, and Blood Institute, during the conduct of the study. Dr Stone has received speaker honoraria from Medtronic, Pulnovo, Abiomed, Amgen, and Boehringer Ingelheim; has served as a consultant to Abbott, Daiichi-Sankyo, Ablative Solutions, CorFlow, Cardiomech, Robocath, Miracor Medical, Vectorious Medical Technologies, Apollo Therapeutics, Elucid Bio, Cardiac Success, Valfix, TherOx, HeartFlow, Neovasc, Ancora, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, Oxitope, HighLife, Elixir, Remote Cardiac Enablement, and Aria; and has equity or options with Cardiac Success, Ancora, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWAVE, Orchestra Biomed, Aria, Valfix, and Xenter. Dr Stone’s employer, Mount Sinai Hospital, receives research grants from Shockwave Medical, Abbott, Abiomed, Bioventrix, Cardiovascular Systems, Phillips, Biosense Webster, Vascular Dynamics, Pulnovo, and V-Wave. Dr O’Brien has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Hochman was the principal investigator for the ISCHEMIA trial, for which, in addition to support from a National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular, Medtronic, Abbott Laboratories (formerly St Jude Medical), Royal Philips NV (formerly Volcano Corporation), Arbor Pharmaceuticals, AstraZeneca Pharmaceuticals, Merck Sharp & Dohme, and Omron Healthcare and financial donations by Arbor Pharmaceuticals and AstraZeneca Pharmaceuticals; and is the principal investigator for ISCHEMIA-EXTEND. Dr Maron received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; and has received personal fees from Abiomed and Regeneron. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






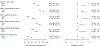
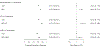
References
-
- Lee SW, Lee PH, Ahn JM et al. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion. Circulation 2019;139:1674–1683. - PubMed
-
- Werner GS, Martin-Yuste V, Hildick-Smith D et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J 2018;39:2484–2493. - PubMed
-
- Mashayekhi K, Nuhrenberg TG, Toma A et al. A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion: The REVASC Trial. JACC Cardiovasc Interv 2018;11:1982–1991. - PubMed
-
- Henriques JP, Hoebers LP, Ramunddal T et al. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients With STEMI: The EXPLORE Trial. Journal of the American College of Cardiology 2016;68:1622–1632. - PubMed
-
- Obedinskiy AA, Kretov EI, Boukhris M et al. The IMPACTOR-CTO Trial. JACC Cardiovasc Interv 2018;11:1309–1311. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

