Nutritional ketosis modulates the methylation of cancer-related genes in patients with obesity and in breast cancer cells
- PMID: 40140215
- PMCID: PMC12279599
- DOI: 10.1007/s13105-025-01076-9
Nutritional ketosis modulates the methylation of cancer-related genes in patients with obesity and in breast cancer cells
Abstract
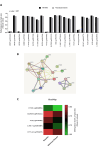
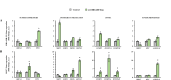
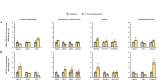
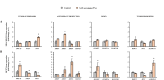
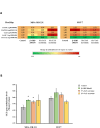
Scientific evidence demonstrates that a very low-calorie ketogenic diet (VLCKD) is effective and beneficial in the treatment of obesity, capable of reversing the methylome associated with obesity and has immunomodulatory capacity. This effect is in part promoted by nutritional ketosis and could be involved in counteracting obesity-related cancer. The aim of this study was to evaluate the effect of nutritional ketosis on the methylation of genes related to tumor processes in patients with obesity and in breast cancer cells. Based on methylome data (Infinium MethylationEPIC BeadChip, Illumina) from patients with obesity treated with a VLCKD for weight loss (n = 10; n = 5 women, age = 48.8 ± 9.20 years, BMI = 32.9 ± 1.4 kg/m2), genes belonging to cancer-related pathways were specifically evaluated and further validated in vitro in MDA-MB-231 (triple negative) and MCF7 (RE positive) breast tumor cells pretreated for 72 h with βOHB, the main ketone body, secretome from visceral (VATs) or subcutaneous (SATs) adipose tissue of patients with obesity. The cell tumoral phenotype was evaluated by proliferation assay and expression of cancer-related genes. VLCKD-induced nutritional ketosis promoted changes in the methylation of 18 genes (20 CpGs; 17 hypomethylated, 3 hypermethylated) belonged to cancer-related pathways with MAPK10, CCN1, CTNNA2, LAMC3 and GLI2 being the most representative genes. A similar pattern was observed in the MDA-MB-231 cells treated with β-OHB, without changes in MCF7. These epigenetic changes paralleled the tumoral phenotype modulated by the treatments. Taking together these results highlight the potential role of VLCKD as an adjuvant to anticancer treatment in groups more susceptible to the development of cancer such as patients with obesity, exerting epigenetic regulation through nutritional ketosis and weight loss.
Keywords: Adipose tissue; Breast cancer; DNMTs; Epigenetics; Ketogenic diet; Ketone bodies; Oncogenes; Sirtuins; Tumor suppressors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: All authors declare to have no competing financial interests in relation to the work described except A.B.C., D. dL and D.B. who received advisory board fees and/or research grants from Pronokal group Spain, a Nestlé Health Science company.
Figures
References
-
- Soerjomataram I, Bray F (2021) Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat Rev Clin Oncol 18(10):663–672 - PubMed
-
- Cabia B, Andrade S, Carreira MC, Casanueva FF, Crujeiras AB (2016) A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes Rev 17(4):361–376 - PubMed
-
- Crujeiras AB, Casanueva FF (2015) Obesity and the reproductive system disorders: epigenetics as a potential Bridge. Hum Reprod Update 21(2):249–261 - PubMed
-
- Crujeiras AB, Cabia B, Carreira MC, Amil M, Cueva J, Andrade S et al (2016) Secreted factors derived from obese visceral adipose tissue regulate the expression of breast malignant transformation genes. Int J Obes 40(3):514–523 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

