External acidity as performance descriptor in polyolefin cracking using zeolite-based materials
- PMID: 40140345
- PMCID: PMC11947190
- DOI: 10.1038/s41467-025-57158-1
External acidity as performance descriptor in polyolefin cracking using zeolite-based materials
Abstract
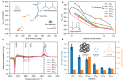
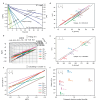
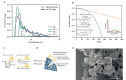
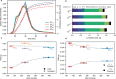
Thermal pyrolysis is gaining industrial adoption to convert large volumes of plastic waste into hydrocarbon feedstock. However, it suffers from a high reaction temperature and relatively low selectivity. Utilizing a catalyst in the process, moving from thermal pyrolysis to catalytic cracking could help overcome both challenges. In order to develop efficient catalyst materials for this process, understanding structure-composition-performance relationships is critical. In this work, we show that in contrast to cracking of small molecules, plastic cracking activity using ultrastable zeolite Y materials does not depend on the bulk Brønsted acid site content, but rather on the concentration of acid sites located on the outer surface and in mesopores. This external acidity, however, fails to capture all the observed performance trends. Detailed kinetic experiments reveal that the scaling of the reaction rate with the catalyst loading differs drastically between highly similar catalyst materials. More specifically, doubling the catalyst loading leads to doubling of the reaction rate for one material, while for another it leads to more than fivefold increase. When very bulky reactants, such as polyolefins, are converted over microporous catalysts, structure-composition-performance relationships established for smaller molecules need to be revisited.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Catalytic Pyrolysis of Polyethylene with Microporous and Mesoporous Materials: Assessing Performance and Mechanistic Understanding.ChemSusChem. 2025 Apr 1;18(7):e202401141. doi: 10.1002/cssc.202401141. Epub 2024 Nov 7. ChemSusChem. 2025. PMID: 39255052 Free PMC article.
-
Processing renewable and waste-based feedstocks with fluid catalytic cracking: Impact on catalytic performance and considerations for improved catalyst design.Front Chem. 2023 Jan 19;11:1067488. doi: 10.3389/fchem.2023.1067488. eCollection 2023. Front Chem. 2023. PMID: 36742037 Free PMC article.
-
Conversion of plastic waste into fuel oil using zeolite catalysts in a bench-scale pyrolysis reactor.RSC Adv. 2022 Mar 8;12(13):7612-7620. doi: 10.1039/d1ra08673a. eCollection 2022 Mar 8. RSC Adv. 2022. PMID: 35424760 Free PMC article.
-
Catalytic Cracking of Liquefied Petroleum Gas (LPG) to Light Olefins Using Zeolite-Based Materials: Recent Advances, Trends, Challenges and Future Perspectives.Chem Rec. 2024 Nov;24(11):e202400110. doi: 10.1002/tcr.202400110. Epub 2024 Nov 7. Chem Rec. 2024. PMID: 39508601 Review.
-
Two-Dimensional Zeolite Materials: Structural and Acidity Properties.Materials (Basel). 2020 Apr 12;13(8):1822. doi: 10.3390/ma13081822. Materials (Basel). 2020. PMID: 32290625 Free PMC article. Review.
References
-
- Levi, P. G. & Cullen, J. M. Mapping global flows of chemicals: From fossil fuel feedstocks to chemical products. Environ. Sci. Technol.52, 1725–1734 (2018). - PubMed
-
- LyondellBasell to Build Industrial-scale Advanced Recycling Plant in Germany. https://www.lyondellbasell.com/en/news-events/corporate--financial-news/... (2023).
-
- Haag, W. O., Lago, R. M. & Weisz, P. B. The active site of acidic aluminosilicate catalysts. Nature309, 589–591 (1984).
Grants and funding
LinkOut - more resources
Full Text Sources

