Cocaine perturbs neurodevelopment and increases neuroinflammation in a prenatal cerebral organoid model
- PMID: 40140359
- PMCID: PMC11947122
- DOI: 10.1038/s41398-025-03315-5
Cocaine perturbs neurodevelopment and increases neuroinflammation in a prenatal cerebral organoid model
Abstract
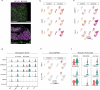
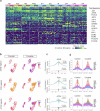
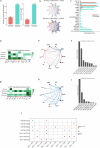
Prenatal exposure to cocaine causes abnormalities in foetal brain development, which are linked to later development of anxiety, depression and cognitive dysfunction. Previous studies in rodent models have indicated that prenatal cocaine exposure affects proliferation, differentiation and connectivity of neural cell types. Here, using cerebral organoids derived from the human iPSC cell line HPSI1213i-babk_2, we investigated cocaine-induced changes of the gene expression regulatory landscape at an early developmental time point, leveraging recent advances in single cell RNA-seq and single cell ATAC-seq. iPSC-cerebral organoids replicated well-established cocaine responses observed in vivo and provided additional information about the cell-type specific regulation of gene expression following cocaine exposure. Cocaine altered gene expression patterns, in part through epigenetic landscape remodelling, and revealed disordered neural plasticity mechanisms in the cerebral organoids. Perturbed neurodevelopmental cellular signalling and an inflammatory-like activation of astrocyte populations were also evident following cocaine exposure. The combination of altered neuroplasticity, neurodevelopment and neuroinflammatory signalling suggests cocaine exposure can mediate substantial disruption of normal development and maturation of the brain. These findings offer new insights into the cellular mechanism underlying the adverse effects of cocaine exposure on neurodevelopment and point to the possible pathomechanisms of later neuropsychiatric disturbances.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Corbett GA, Carmody D, Rochford M, Cunningham O, Lindow SW, O’Connell MP. Drug use in pregnancy in Ireland’s capital city: a decade of trends and outcomes. Eur J Obstet Gynecol Reprod Biol. 2023;282:24–30. 10.1016/j.ejogrb.2022.12.021 - PubMed
-
- Bandstra ES, Morrow CE, Accornero VH, Mansoor E, Xue L, Anthony JC. Estimated effects of in utero cocaine exposure on language development through early adolescence. Neurotoxicol Teratol. 2011;33:25–35. 10.1016/j.ntt.2010.07.001 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

