Helminth driven gut inflammation and microbial translocation associate with altered vaccine responses in rural Uganda
- PMID: 40140378
- PMCID: PMC11947158
- DOI: 10.1038/s41541-025-01116-x
Helminth driven gut inflammation and microbial translocation associate with altered vaccine responses in rural Uganda
Abstract
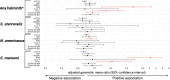
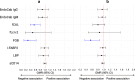
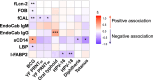
Vaccine responses are sometimes impaired in rural, low-income settings. Helminth-associated gut barrier dysfunction and microbial translocation (MT) may be implicated. We used samples from a trial of praziquantel treatment-effects on vaccine responses in Schistosoma mansoni (Sm)-endemic Ugandan islands, measuring intestinal fatty acid-binding protein 2 (I-FABP2), lipopolysaccharide-binding protein, anti-endotoxin core antibodies (EndoCab), soluble CD14 (sCD14) in plasma, and faecal lipocalin-2, occult blood (FOB), and calprotectin (fCAL), and evaluating their associations with baseline helminth infection, praziquantel treatment, and responses to BCG, yellow fever, typhoid, HPV, and tetanus-diphtheria vaccines. Sm associated positively with fCAL and FOB, hookworm with I-FABP2, and any helminth with EndoCab IgM, fCAL and FOB. Sm associated inversely with sCD14. Praziquantel treatment reduced all marker concentrations, significantly fCAL and FOB, implying that Sm-associated gut inflammation and MT is reversible. Associations of assessed markers with vaccine-specific responses were predominantly inverse. Interventions to improve gut barrier function may enhance vaccine responsiveness.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.N. and A.M.E. report grants from Wellcome Trust. G.N. reports funding from the EDCTP2 programme supported by the European Union. A.M.E. reports funding from Medical Research Council (MRC) of the United Kingdom for conduct of the parent study; A.M.E. reports funding from NIH, Science for Africa Foundation, the Royal Society, and DELTAS Africa, outside the submitted work. A.M.E. and A.N. report support from UK National Institute of Health and care Research (NIHR). A.M.E. further reports support from the Serum Institute of India, Uganda National Expanded Programme on Immunisation, and Emergent BioSolutions for conduct of the parent study. All other authors declare no competing interests.
Figures

References
-
- Barreto, M. L. et al. Causes of variation in BCG vaccine efficacy: Examining evidence from the BCG REVAC cluster randomized trial to explore the masking and the blocking hypotheses. Vaccine32, 3759–3764 (2014). - PubMed
-
- Black, G. F. et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet359, 1393–1401 (2002). - PubMed
-
- Fine, P. E. M. Variation in protection by BCG: implications of and for heterologous immunity. Lancet346, 1339–1345 (1995). - PubMed
Grants and funding
- DEL-15-004/DELTAS Africa Initiative
- DEL-15-004/DELTAS Africa Initiative
- R120442/Royal Society
- R120442/Royal Society
- NIHR134531/National Institute for Health and Care Research
- NIHR134531/National Institute for Health and Care Research
- NIHR134531/National Institute for Health and Care Research
- NIHR134531/National Institute for Health and Care Research
- MR/R010161/1/MRC_/Medical Research Council/United Kingdom
- MR/R02118X/1/MRC_/Medical Research Council/United Kingdom
- TMA2019PF-2707/European and Developing Countries Clinical Trials Partnership
- 224263/Z/21/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials

