Non-muscle myosin II inhibition at the site of axon injury increases axon regeneration
- PMID: 40140393
- PMCID: PMC11947156
- DOI: 10.1038/s41467-025-58303-6
Non-muscle myosin II inhibition at the site of axon injury increases axon regeneration
Abstract
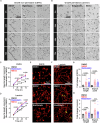
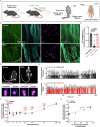
Motor axon regeneration following peripheral nerve injury is critical for motor recovery but therapeutic interventions enhancing this are not available. We conduct a phenotypic screen on human motor neurons and identified blebbistatin, a non-muscle myosin II inhibitor, as the most effective neurite outgrowth promotor. Despite its efficacy in vitro, its poor bioavailability limits in vivo application. We, therefore, utilize a blebbistatin analog, NMIIi2, to explore its therapeutic potential for promoting axon regeneration. Local NMIIi2 application directly to injured axons enhances regeneration in human motor neurons. Furthermore, following a sciatic nerve crush injury in male mice, local NMIIi2 administration to the axonal injury site facilitates motor neuron regeneration, muscle reinnervation, and functional recovery. NMIIi2 also promotes axon regeneration in sensory, cortical, and retinal ganglion neurons. These findings highlight the therapeutic potential of topical NMII inhibition for treating axon damage.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.J.W. is a founder of Nocion Therapeutics, QurAlis, and BlackBox Bio and is on the scientific advisory board of Lundbeck Pharma, Axonis, and Tafalgie Therapeutics. Z.H. is a co-founder of Rugen and Myrobalan, and an advisor of Axonis. The remaining authors declare no competing interests.
Figures




References
-
- Lundborg, G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J. Hand Surg. Am.25, 391–414 (2000). - PubMed
-
- Novak, C. B., Anastakis, D. J., Beaton, D. E., Mackinnon, S. E. & Katz, J. Biomedical and psychosocial factors associated with disability after peripheral nerve injury. J. Bone Jt. Surg. Am.93, 929–936 (2011). - PubMed

