Evidence supporting a catalytic pentad mechanism for the proteasome and other N-terminal nucleophile enzymes
- PMID: 40140419
- PMCID: PMC11947121
- DOI: 10.1038/s41467-025-58077-x
Evidence supporting a catalytic pentad mechanism for the proteasome and other N-terminal nucleophile enzymes
Abstract
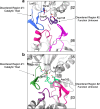
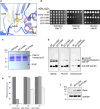
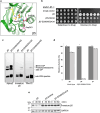
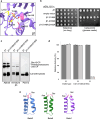
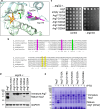
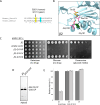
Proteases are defined by their nucleophile but require additional residues to regulate their active sites, most often arranged as catalytic triads that control the generation and resolution of acyl-enzyme intermediates. Threonine N-terminal nucleophiles represent a diverse family of proteases and transferases that possess two active site nucleophiles, the side chain hydroxyl and the free amino-terminus, and require autocatalytic cleavage of their N-terminal propeptides. Here we provide evidence that the proteasome, which mediates intracellular protein degradation and contains three different threonine protease subunits, utilizes a unique catalytic pentad mechanism. In addition to the previously defined lysine/aspartate pair which regulates threonine's side chain, a second serine/aspartate pair appears to regulate threonine's amino-terminus. The pentad is required for substrate proteolysis and assembly-coupled autocatalytic cleavage, the latter triggered by alignment of the full pentad upon fusion of two half-proteasome precursors. A similar pentad mechanism was required by the ornithine acetyltransferase Arg7, suggesting that this may be a general property of threonine N-terminal nucleophiles. Finally, we show that two patient-derived proteasome mutations compromise function of the serine/aspartate unit in yeast, suggesting that defective pentad function may underlie some human proteasomopathies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Brannigan, J. A. et al. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature378, 416–419 (1995). - PubMed
-
- Seemuller, E. et al. Proteasome from thermoplasma acidophilum: a threonine protease. Science268, 579–582 (1995). - PubMed
-
- Seemuller, E., Lupas, A. & Baumeister, W. Autocatalytic processing of the 20S proteasome. Nature382, 468–471 (1996). - PubMed
-
- Groll, M. et al. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature386, 463–471 (1997). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

