Longitudinal fecal microbiota and volatile metabolomics preceding necrotizing enterocolitis in preterm infants: a case-control study
- PMID: 40140438
- PMCID: PMC11947453
- DOI: 10.1038/s41598-025-94692-w
Longitudinal fecal microbiota and volatile metabolomics preceding necrotizing enterocolitis in preterm infants: a case-control study
Erratum in
-
Author Correction: Longitudinal fecal microbiota and volatile metabolomics preceding necrotizing enterocolitis in preterm infants: a case-control study.Sci Rep. 2025 Jul 28;15(1):27455. doi: 10.1038/s41598-025-11378-z. Sci Rep. 2025. PMID: 40721450 Free PMC article. No abstract available.
Abstract
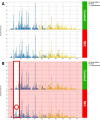
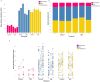
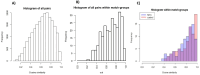
Alterations in fecal microbiota and volatile organic compound (VOC) profiles of preterm infants have been demonstrated before onset of necrotizing enterocolitis (NEC). However, NEC-specific signatures need to be identified before potential application as predictive biomarker in clinical practice. A prospective multicenter case-control study was conducted to identify preclinical fecal microbiota and VOC profiles of infants that developed NEC. Microbiota analysis (PCR-based IS-pro technique) and VOC analysis (gas chromatography-mass spectrometry) were performed on fecal samples collected up to three days before clinical NEC onset. In 112 infants (56 NEC, 56 matched controls), sufficient number fecal samples were collected for either microbiota or VOC analysis. Prior to NEC onset, Clostridium perfringens (p = 0.023, unadjusted) was more present in infants with NEC, versus controls. VOC analysis showed a clear distinction between fecal profiles of NEC cases and controls (area under the curve = 0.82). Fourteen unique VOC features contributed to this discrimination. Fecal microbiota and VOC profiles may serve as early indicators of NEC, and allow for increased understanding of pathophysiological mechanisms of NEC, but larger validation cohorts are needed before an overarching NEC-specific predictive microbiota-based biomarker can be implemented.
Keywords: Metabolomics; Microbiome; Necrotizing enterocolitis; Stool; Volatile organic compounds.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics statement: The study was approved by all local Medical Ethical Review Boards (protocol number A2016.313) and written informed consent was obtained from the legal guardians of all included infants. All experiments were performed in accordance with relevant guidelines and regulations.
Figures


References
-
- Thyoka, M. et al. Advanced necrotizing enterocolitis part 1: Mortality. Eur. J. Pediatric Surg.22(1), 8–12 (2012). - PubMed
-
- Eaton, S., Rees, C. M. & Hall, N. J. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology111(4), 423–430 (2017). - PubMed
-
- Rich, B. S. & Dolgin, S. E. Necrotizing enterocolitis. Pediatr. Rev.38(12), 552–559 (2017). - PubMed
-
- Neu, J. Prevention of necrotizing enterocolitis. Clin. Perinatol.49(1), 195–206 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

