A small-molecule SARS-CoV-2 inhibitor targeting the membrane protein
- PMID: 40140563
- PMCID: PMC11981937
- DOI: 10.1038/s41586-025-08651-6
A small-molecule SARS-CoV-2 inhibitor targeting the membrane protein
Abstract
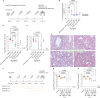
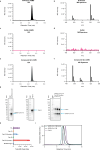
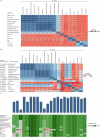
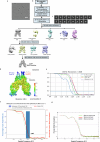
The membrane (M) protein of betacoronaviruses is well conserved and has a key role in viral assembly1,2. Here we describe the identification of JNJ-9676, a small-molecule inhibitor targeting the coronavirus M protein. JNJ-9676 demonstrates in vitro nanomolar antiviral activity against SARS-CoV-2, SARS-CoV and sarbecovirus strains from bat and pangolin zoonotic origin. Using cryogenic electron microscopy (cryo-EM), we determined a binding pocket of JNJ-9676 formed by the transmembrane domains of the M protein dimer. Compound binding stabilized the M protein dimer in an altered conformational state between its long and short forms, preventing the release of infectious virus. In a pre-exposure Syrian golden hamster model, JNJ-9676 (25 mg per kg twice per day) showed excellent efficacy, illustrated by a significant reduction in viral load and infectious virus in the lung by 3.5 and 4 log10-transformed RNA copies and 50% tissue culture infective dose (TCID50) per mg lung, respectively. Histopathology scores at this dose were reduced to the baseline. In a post-exposure hamster model, JNJ-9676 was efficacious at 75 mg per kg twice per day even when added at 48 h after infection, when peak viral loads were observed. The M protein is an attractive antiviral target to block coronavirus replication, and JNJ-9676 represents an interesting chemical series towards identifying clinical candidates addressing the current and future coronavirus pandemics.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.V.L., E.V.D., M.V.G. and C.B. are named as inventors on a pending patent application claiming inhibitors of coronavirus (WO 2024/008909), which was filed by the Applicant Janssen Pharmaceutica. M.V.L., E.V.D., J.X., S.J., L.G., J.D., M.V.G., R.D.-A., A.D., S. Marsili, S. Miller, C.V.d.E., A.D.R., P.V., K.T., D.P. and C.B. were/are employees of Janssen Pharmaceutica and may possess stocks of Johnson & Johnson. S. Miller is an employee of Spark Therapeutics and may possess stocks of Roche. N.V.d.B., L.T., V.R. and I.D.P. are employees of Charles River Laboratories, a contract research organization and may possess stocks of Johnson & Johnson. M.S. is an employee of Gilead Sciences and may possess stocks of Gilead Sciences. A.A.L., H.L.M.d.G., E.J.S. and M.J.v.H. received funding from Janssen Pharmaceutica to perform contract research. M.D.T., M.Z. and F.J.M.v.K. received funding from Janssen Pharmaceutica to perform contract research. The other authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

