Changes in neurotensin signalling drive hedonic devaluation in obesity
- PMID: 40140571
- PMCID: PMC12119351
- DOI: 10.1038/s41586-025-08748-y
Changes in neurotensin signalling drive hedonic devaluation in obesity
Abstract
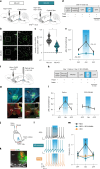
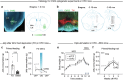
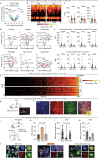
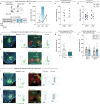
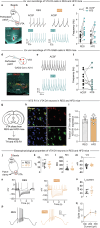
Calorie-rich foods, particularly those that are high in fat and sugar, evoke pleasure in both humans and animals1. However, prolonged consumption of such foods may reduce their hedonic value, potentially contributing to obesity2-4. Here we investigated this phenomenon in mice on a chronic high-fat diet (HFD). Although these mice preferred high-fat food over regular chow in their home cages, they showed reduced interest in calorie-rich foods in a no-effort setting. This paradoxical decrease in hedonic feeding has been reported previously3-7, but its neurobiological basis remains unclear. We found that in mice on regular diet, neurons in the lateral nucleus accumbens (NAcLat) projecting to the ventral tegmental area (VTA) encoded hedonic feeding behaviours. In HFD mice, this behaviour was reduced and uncoupled from neural activity. Optogenetic stimulation of the NAcLat→VTA pathway increased hedonic feeding in mice on regular diet but not in HFD mice, though this behaviour was restored when HFD mice returned to a regular diet. HFD mice exhibited reduced neurotensin expression and release in the NAcLat→VTA pathway. Furthermore, neurotensin knockout in the NAcLat and neurotensin receptor blockade in the VTA each abolished optogenetically induced hedonic feeding behaviour. Enhancing neurotensin signalling via overexpression normalized aspects of diet-induced obesity, including weight gain and hedonic feeding. Together, our findings identify a neural circuit mechanism that links the devaluation of hedonic foods with obesity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

