Connectome-driven neural inventory of a complete visual system
- PMID: 40140576
- PMCID: PMC12119369
- DOI: 10.1038/s41586-025-08746-0
Connectome-driven neural inventory of a complete visual system
Abstract
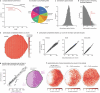
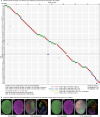
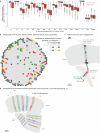
Vision provides animals with detailed information about their surroundings and conveys diverse features such as colour, form and movement across the visual scene. Computing these parallel spatial features requires a large and diverse network of neurons. Consequently, from flies to humans, visual regions in the brain constitute half its volume. These visual regions often have marked structure-function relationships, with neurons organized along spatial maps and with shapes that directly relate to their roles in visual processing. More than a century of anatomical studies have catalogued in detail cell types in fly visual systems1-3, and parallel behavioural and physiological experiments have examined the visual capabilities of flies. To unravel the diversity of a complex visual system, careful mapping of the neural architecture matched to tools for targeted exploration of this circuitry is essential. Here we present a connectome of the right optic lobe from a male Drosophila melanogaster acquired using focused ion beam milling and scanning electron microscopy. We established a comprehensive inventory of the visual neurons and developed a computational framework to quantify their anatomy. Together, these data establish a basis for interpreting how the shapes of visual neurons relate to spatial vision. By integrating this analysis with connectivity information, neurotransmitter identity and expert curation, we classified the approximately 53,000 neurons into 732 types. These types are systematically described and about half are newly named. Finally, we share an extensive collection of split-GAL4 lines matched to our neuron-type catalogue. Overall, this comprehensive set of tools and data unlocks new possibilities for systematic investigations of vision in Drosophila and provides a foundation for a deeper understanding of sensory processing.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




















Update of
-
Connectome-driven neural inventory of a complete visual system.bioRxiv [Preprint]. 2024 Jun 1:2024.04.16.589741. doi: 10.1101/2024.04.16.589741. bioRxiv. 2024. Update in: Nature. 2025 May;641(8065):1225-1237. doi: 10.1038/s41586-025-08746-0. PMID: 38659887 Free PMC article. Updated. Preprint.
References
-
- Ramón y Cajal, S. & Sánchez, D. Contribución al conocimiento de los centros nerviosos de los insectos (Imprenta de Hijos de Nicolás Moya, 1915).
-
- Strausfeld, N. J. Atlas of an Insect Brain (Springer, 1976).
-
- Fischbach, K.-F. & Dittrich, A. P. M. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res.258, 441–475 (1989).
-
- Meinertzhagen, I. A. & O’Neil, S. D. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J. Comp. Neurol.305, 232–263 (1991). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

