Gene-modified pig-to-human liver xenotransplantation
- PMID: 40140580
- PMCID: PMC12095057
- DOI: 10.1038/s41586-025-08799-1
Gene-modified pig-to-human liver xenotransplantation
Abstract
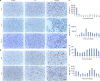
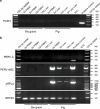
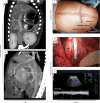
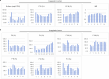
The shortage of donors is a major challenge for transplantation; however, organs from genetically modified pigs can serve as ideal supplements1,2. Until now, porcine hearts and kidneys have been successively transplanted into humans3-7. In this study, heterotopic auxiliary transplantation was used to donate a six-gene-edited pig liver to a brain-dead recipient. The graft function, haemodynamics, and immune and inflammatory responses of the recipient were monitored over the subsequent 10 days. Two hours after portal vein reperfusion of the xenograft, goldish bile was produced, increasing to 66.5 ml by postoperative day 10. Porcine liver-derived albumin also increased after surgery. Alanine aminotransferase levels remained in the normal range, while aspartate aminotransferase levels increased on postoperative day 1 and then rapidly declined. Blood flow velocity in the porcine hepatic artery and portal and hepatic veins remained at an acceptable level. Although platelet numbers decreased early after surgery, they ultimately returned to normal levels. Histological analyses showed that the porcine liver regenerated capably with no signs of rejection. T cell activity was inhibited by anti-thymocyte globulin administration, and B cell activation increased 3 days after surgery and was then inhibited by rituximab. There were no significant peri-operative changes in immunoglobulin G or immunoglobulin M levels. C-reactive protein and procalcitonin levels were initially elevated and then quickly declined. The xenograft remained functional until study completion.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Pan, D. et al. Progress in multiple genetically modified minipigs for xenotransplantation in China. Xenotransplantation26, e12492 (2019). - PubMed
-
- Montgomery, R. A. et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med.386, 1889–1898 (2022). - PubMed
-
- Loupy, A. et al. Immune response after pig-to-human kidney xenotransplantation: a multimodal phenotyping study. Lancet402, 1158–1169 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

