Understanding and Managing Infusion Reactions and Hypophosphataemia With Intravenous Iron-A Nurses' Consensus Paper
- PMID: 40140601
- PMCID: PMC11946542
- DOI: 10.1002/nop2.70191
Understanding and Managing Infusion Reactions and Hypophosphataemia With Intravenous Iron-A Nurses' Consensus Paper
Abstract
Aim: To provide evidence-based guidance on practical aspects and potential safety concerns (infusion reactions and hypophosphataemia) related to the use of intravenous iron from a nursing perspective.
Design: A modified Delphi consensus method.
Methods: Literature searches were conducted and used to support the development of 16 consensus statements. Six nurses with expertise in the field of gastroenterology and experience with the administration of intravenous iron participated in a modified Delphi process to develop a final set of statements.
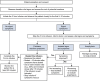
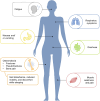
Results: Overall, 16 statements achieved consensus and covered the practicalities of administration, infusion reactions and hypophosphataemia. Patient preparation is a key step in the administration of intravenous iron, but information should be communicated carefully to prevent undue anxiety. Highlighting the nurse's confidence in the management of any reactions may help to reduce anxiety. The patient should be observed during the first 5-10 min of an infusion to allow prompt management of immediate infusion reactions, although severe hypersensitivity reactions are rare. Nurses should be vigilant for symptoms of hypophosphataemia (such as fatigue, weakness and muscle/bone pain), which can develop following treatment with ferric carboxymaltose, saccharated ferric oxide and iron polymaltose. Serum phosphate levels should be measured in patients receiving ferric carboxymaltose who are at risk of low phosphate.
Impact: Infusion reactions and hypophosphataemia with intravenous iron are documented in the literature, but existing publications do not approach these topics from a nursing perspective. This consensus paper highlights the importance of patient preparation, monitoring and prompt management when administering intravenous iron to ensure patient safety. Considering that nurses have a central role in the administration of intravenous iron, the availability of evidence-based guidance is essential for both nurse confidence and patient safety.
Patient or public contribution: No patient or public contribution was involved in the consensus process.
Keywords: fatigue; hypersensitivity reactions; hypophosphataemia; intravenous iron; nursing; safety.
© 2025 The Author(s). Nursing Open published by John Wiley & Sons Ltd.
Conflict of interest statement
A.F. has been a consultant and speaker for Abbvie Ltd. Dr Falk Pharma, Galapagos, Gilead, Janssen, Pfizer, Pharmacosmos, Takeda UK Ltd. and Tillotts. V.C. has been a consultant and speaker for Falk, Ferring, Janssen, Pharmacosmos and Takeda. C.K. has been a consultant and speaker for Abbvie, Pharmacosmos, Takeda and Vifor Pharma. M.A. has been a consultant and speaker for Abbvie, Janssen, Pharmacosmos, Takeda and Tillotts. E.M. and R.L. declare no conflicts of interest.
Figures
References
-
- Achebe, M. O. , Mandell E., Jolley K., et al. 2023. “Pagophagia and Restless Legs Syndrome Are Highly Associated With Iron Deficiency and Should Be Included in Histories Evaluating Anemia.” American Journal of Hematology 98, no. 1: E8–E10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

