Inhibition of 6-phosphogluconate dehydrogenase suppresses esophageal squamous cell carcinoma growth and enhances the anti-tumor effects of metformin via the AMPK/mTOR pathway
- PMID: 40140842
- PMCID: PMC11938747
- DOI: 10.1186/s12943-025-02302-0
Inhibition of 6-phosphogluconate dehydrogenase suppresses esophageal squamous cell carcinoma growth and enhances the anti-tumor effects of metformin via the AMPK/mTOR pathway
Abstract
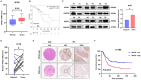
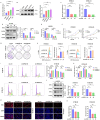
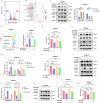
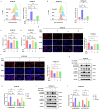
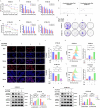
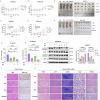
Metabolic reprogramming plays a pivotal role in the development and progression of tumors. Tumor cells rely on glycolysis as their primary energy production pathway and effectively utilize biomolecules generated by the pentose phosphate pathway (PPP) for efficient biosynthesis. However, the role of 6-phosphogluconate dehydrogenase (6PGD), a crucial enzyme in the PPP, remains unexplored in esophageal squamous cell carcinoma (ESCC). In this study, we observed a significant upregulation of 6PGD expression in ESCC tissues, which correlated with an unfavorable prognosis among patients. The experiments demonstrated that knockdown of 6PGD induces oxidative stress and suppresses ESCC cell proliferation. Mechanistically, this is achieved through AMPK activation and subsequent inhibition of downstream mTOR phosphorylation. Moreover, physcion has been found to inhibit 6PGD activity and exert its anti-ESCC effect via the AMPK/mTOR pathway. Subsequently, we conducted both in vitro and in vivo experiments to validate the anticancer efficacy of combining metformin, an AMPK activator, with physcion. The results demonstrated a significantly enhanced inhibition of ESCC growth. This study elucidates the impact of 6PGD on ESCC cell proliferation along with its underlying molecular mechanisms, highlighting its potential as a therapeutic target for ESCC. Furthermore, we investigated a novel approach for improved anti-tumor therapy involving physcion and metformin. These findings will contribute new insights to clinical treatment strategies for ESCC while providing a theoretical foundation for developing molecular targeted therapies.
Keywords: 6-phosphogluconate dehydrogenase; Esophageal squamous cell carcinoma; Metformin; Pentose phosphate pathway; Physcion.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study was conducted with the approval of the Ethics Committee of the Yancheng First People’s Hospital (Approval number: 2022-K-016), and all patients provided written informed consent. The animal experimental procedures strictly adhered to the guidelines for animal welfare and were approved by the Ethics Committee for Medical Experimental Animals of Jiangsu Medical College (Approval number: XMLL-2022-853).
Figures


References
-
- Zhu H, et al. Esophageal cancer in China: practice and research in the new era. Int J Cancer. 2023;152(9):1741–51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

