Investigator-initiated versus industry-sponsored trials - visibility and relevance of randomized controlled trials in clinical practice guidelines (IMPACT)
- PMID: 40140983
- PMCID: PMC11948659
- DOI: 10.1186/s12874-025-02535-z
Investigator-initiated versus industry-sponsored trials - visibility and relevance of randomized controlled trials in clinical practice guidelines (IMPACT)
Abstract
Background: The goal of evidence-based medicine is to make clinical decisions based on the best available, relevant evidence. For this to be possible, studies such as randomized controlled trials (RCTs), which are widely considered to provide the best evidence of all forms of primary research, must be visible and have an impact on clinical practice guidelines. We further investigated the impact of publicly and commercially sponsored RCTs on clinical practice guidelines by measuring direct and indirect impactful citations and the time to guideline impact.
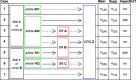
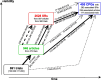
Methods: We considered the sample from the IMPACT study, where a total of 691 RCTs (120 German investigator-initiated trials (IITs), 200 international IITs, 171 German industry-sponsored trials (ISTs) and 200 international ISTs) was sampled from registries (DFG-/BMBF-Websites, the German Clinical Trials Register, and from ClinicalTrials.gov) and followed prospectively. First, all eligible IITs were sampled. Then, ISTs were randomly selected while ensuring balance across certain trial characteristics. Next, the corresponding publications in the form of original research articles were identified. A search was then conducted for (1) systematic reviews (SRs) citing these articles and (2) clinical practice guidelines (CPGs) that cited either the original articles or the SRs. The methods and results of this effort were already published. In this investigation we aimed to better characterize the impact of RCTs in CPGs. Therefore, we identified all citations of the original articles and SRs in the citing CPGs and classified them into impactful and non-impactful. This allowed us to calculate an estimate for the guideline impact of a trial. In addition, we estimated the time-to-guideline-impact, defined as the time to a direct and indirect impactful citation in a CPG. Direct means that the publication of a trial was cited in the main text of a CPG. Indirect means that the publication was cited and included in the findings of a SR and the SR was cited in the main text of a CPG. We also investigated to what extent pre-defined study characteristics influenced the guideline impact using multivariable negative binomial regression as well as the time-to-guideline impact using multivariable Cox proportional hazards regression.
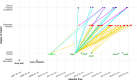
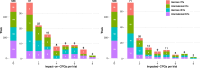
Results: Overall, 22% of RCTs impacted a CPG. For international ISTs, only 15% of trials had an impact in CPGs. Overall, of the 405 associated guidelines, 331 were impacted. Larger trials were associated with more impactful main text citations in CPGs and earlier time-to-guideline impact, while international industry-sponsored trials were associated with smaller impact on CPGs and longer time-to-guideline impact. IITs funded by governmental bodies in Germany reached an impact on CPGs that is on par with German ISTs or international IITs and ISTs.
Conclusion: This study demonstrated that a considerable number of trials previously identified as being linked to CPGs have had impact in those CPGs (85%). International ISTs seem to have a lower impact on CPGs, and fewer of them influence CPGs at all.
Keywords: Access to information; Clinical decision-making; Evidence-based medicine; Health impact assessment; Knowledge translation; Practice guidelines as topic; Publishing; Randomized controlled trials as topic; Registries.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Investigator initiated trials versus industry sponsored trials - translation of randomized controlled trials into clinical practice (IMPACT).BMC Med Res Methodol. 2021 Aug 31;21(1):182. doi: 10.1186/s12874-021-01359-x. BMC Med Res Methodol. 2021. PMID: 34465296 Free PMC article.
-
Impact of investigator initiated trials and industry sponsored trials on medical practice (IMPACT): rationale and study design.BMC Med Res Methodol. 2020 Oct 2;20(1):246. doi: 10.1186/s12874-020-01125-5. BMC Med Res Methodol. 2020. PMID: 33008297 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Guideline-based quality indicators-a systematic comparison of German and international clinical practice guidelines.Implement Sci. 2019 Jul 9;14(1):71. doi: 10.1186/s13012-019-0918-y. Implement Sci. 2019. PMID: 31288828 Free PMC article. Review.
References
-
- Speich B, Von Niederhäusern B, Schur N, Hemkens LG, Fürst T, Bhatnagar N, et al. Systematic review on costs and resource use of randomized clinical trials shows a lack of transparent and comprehensive data. J Clin Epidemiol. 2018;96:1–11. 10.1016/j.jclinepi.2017.12.018. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

