SUMO-G5C23-D208G@ZIF-F: A Novel Immobilized Enzyme with Enhanced Stability and Reusability for Organophosphorus Hydrolysis
- PMID: 40141113
- PMCID: PMC11942619
- DOI: 10.3390/ijms26062469
SUMO-G5C23-D208G@ZIF-F: A Novel Immobilized Enzyme with Enhanced Stability and Reusability for Organophosphorus Hydrolysis
Abstract
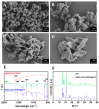
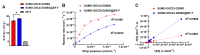
Organophosphorus hydrolase (OPH) is a highly effective bioscavenger for detoxifying hazardous organophosphorus compounds. However, its practical application is hindered by low yield and poor stability. In this study, we employed Small Ubiquitin-like Modifier (SUMO) fusion expression to enhance the solubility of the OPH mutant G5C23-D208G and, for the first time, immobilized the enzyme on a zeolitic imidazolate framework-F (ZIF-F) carrier to improve its stability. The SUMO-G5C23-D208G fusion protein was successfully expressed in Escherichia coli, resulting in a yield that was 2.4 times higher than that of native OPH and an 11-fold increase in solubility. The purified protein achieved a purity of 95%. The immobilized enzyme, SU-MO-G5C23-D208G@ZIF-F, exhibited a farfalle-shaped structure with a diameter of approximately 3-5 μm. Compared to the free enzyme, the immobilized enzyme maintained high catalytic efficiency (kcat/Km = 8.9 × 104 M-1·s-1) and demonstrated enhanced thermal stability, pH stability, and reusability. This study has significantly improved the yield and stability of OPH, thereby supporting its potential for industrial applications.
Keywords: SUMO fusion expression; ZIF-F; enzyme immobilization; enzyme kinetic parameters; organophosphorus hydrolase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

