Gut Microbiota Serves as a Crucial Independent Biomarker in Inflammatory Bowel Disease (IBD)
- PMID: 40141145
- PMCID: PMC11942158
- DOI: 10.3390/ijms26062503
Gut Microbiota Serves as a Crucial Independent Biomarker in Inflammatory Bowel Disease (IBD)
Abstract
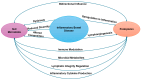
Inflammatory bowel disease (IBD), encompassing Crohn's disease (CD), ulcerative colitis (UC), and IBD unclassified (IBD-U), is a complex intestinal disorder influenced by genetic, environmental, and microbial factors. Recent evidence highlights the gut microbiota as a pivotal biomarker and modulator in IBD pathogenesis. Dysbiosis, characterized by reduced microbial diversity and altered composition, is a hallmark of IBD. A consistent decrease in anti-inflammatory bacteria, such as Faecalibacterium prausnitzii, and an increase in pro-inflammatory species, including Escherichia coli, have been observed. Metabolomic studies reveal decreased short-chain fatty acids (SCFAs) and secondary bile acids, critical for gut homeostasis, alongside elevated pro-inflammatory metabolites. The gut microbiota interacts with host immune pathways, influencing morphogens, glycosylation, and podoplanin (PDPN) expression. The disruption of glycosylation impairs mucosal barriers, while aberrant PDPN activity exacerbates inflammation. Additionally, microbial alterations contribute to oxidative stress, further destabilizing intestinal barriers. These molecular and cellular disruptions underscore the role of the microbiome in IBD pathophysiology. Emerging therapeutic strategies, including probiotics, prebiotics, and dietary interventions, aim to restore microbial balance and mitigate inflammation. Advanced studies on microbiota-targeted therapies reveal their potential to reduce disease severity and improve patient outcomes. Nevertheless, further research is needed to elucidate the bidirectional interactions between the gut microbiome and host immune responses and to translate these insights into clinical applications. This review consolidates current findings on the gut microbiota's role in IBD, emphasizing its diagnostic and therapeutic implications, and advocates for the continued exploration of microbiome-based interventions to combat this debilitating disease.
Keywords: Crohn’s disease; glycosylation; gut microbiota; inflammatory bowel disease; morphogen; podoplanin; ulcerative colitis.
Conflict of interest statement
The authors have no competing interests to declare.
Figures

References
-
- Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., Andrews E., Ajami N.J., Bonham K.S., Brislawn C.J., et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

