Valproic Acid Improves Antisense-Mediated Exon-Skipping Efficacy in mdx Mice
- PMID: 40141224
- PMCID: PMC11942597
- DOI: 10.3390/ijms26062583
Valproic Acid Improves Antisense-Mediated Exon-Skipping Efficacy in mdx Mice
Abstract
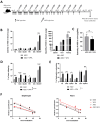
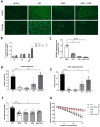
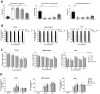
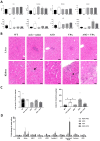
Duchenne muscular dystrophy (DMD) is a severe genetic disorder characterized by the progressive degeneration of skeletal and cardiac muscles due to the absence of dystrophin. Exon-skipping therapy is among the most promising approaches for treating DMD, with several antisense oligonucleotides (ASO) already approved by the FDA; however, their limited efficacy highlights substantial potential for further improvement. In this study, we evaluate the potential of combining ASO with valproic acid (VPA) to enhance dystrophin expression and improve functional outcomes in a murine model of DMD. Our results indicate that the ASO+VPA treatment significantly increases dystrophin restoration across various muscle tissues, with particularly pronounced effects observed in cardiac muscle, where levels are nearly doubled compared to ASO monotherapy. Additionally, we demonstrate significant improvements in functional outcomes in treated mdx mice. Our findings suggest that the combined ASO+VPA therapy holds promise as an effective therapeutic approach to ameliorate muscle function in DMD, warranting further exploration of its mechanistic pathways and long-term benefits.
Keywords: RNA; antisense oligonucleotides; duchenne muscular dystrophy; exon skipping; histone deacetylase inhibitors; transcript imbalance; valproic acid.
Conflict of interest statement
L.G. is co-founder of SQY Therapeutics, which produces tricyclo-DNA oligomers. T.T. was an employee of SQY Therapeutics when performing this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hammond S.M., Aartsma-Rus A., Alves S., Borgos S.E., Buijsen R.A.M., Collin R.W.J., Covello G., Denti M.A., Desviat L.R., Echevarría L., et al. Delivery of Oligonucleotide-Based Therapeutics: Challenges and Opportunities. EMBO Mol. Med. 2021;13:e13243. doi: 10.15252/emmm.202013243. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

