Methodological and Ethical Considerations in the Use of Chordate Embryos in Biomedical Research
- PMID: 40141265
- PMCID: PMC11941781
- DOI: 10.3390/ijms26062624
Methodological and Ethical Considerations in the Use of Chordate Embryos in Biomedical Research
Abstract
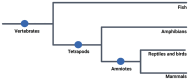
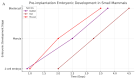
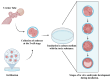
Animal embryos are vital tools in scientific research, providing insights into biological processes and disease mechanisms. This paper explores their historical and contemporary significance, highlighting the shift towards the refinement of in vitro systems as alternatives to animal experimentation. We have conducted a data review of the relevant literature on the use of embryos in research and synthesized the data to highlight the importance of this model for scientific progress and the ethical considerations and regulations surrounding embryo research, emphasizing the importance of minimizing animal suffering while promoting scientific progress through the principles of replacement, reduction, and refinement. Embryos from a wide range of species, including mammals, fish, birds, amphibians, and reptiles, play a crucial experimental role in enabling us to understand factors such as substance toxicity, embryonic development, metabolic pathways, physiological processes, etc., that contribute to the advancement of the biological sciences. To apply this model effectively, it is essential to match the research objectives with the most appropriate methodology, ensuring that the chosen approach is appropriate for the scope of the study.
Keywords: 3Rs; alternatives methods; animal embryos; animal ethics; biomedical research; embryonic development; genetic; model embryos.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Veissier I., Aubert A., Boissy A. Animal welfare: A result of animal background and perception of its environment. Anim. Front. 2012;2:7–15. doi: 10.2527/af.2012-0043. - DOI
-
- McCausland C. The Five Freedoms of Animal Welfare are Rights. J. Agric. Environ. Ethics. 2014;27:649–662. doi: 10.1007/s10806-013-9483-6. - DOI
-
- Kim K., Jo W., Lee G.H. Why do we always care about the welfare of laboratory animals? Open Access Gov. 2023;2023:518–519. doi: 10.56367/OAG-36-10808. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

