The Protective Effects of Burdock Fructooligosaccharide on Preterm Labor Through Its Anti-Inflammatory Action
- PMID: 40141301
- PMCID: PMC11942195
- DOI: 10.3390/ijms26062659
The Protective Effects of Burdock Fructooligosaccharide on Preterm Labor Through Its Anti-Inflammatory Action
Abstract
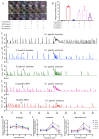
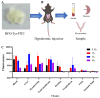
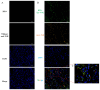
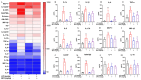
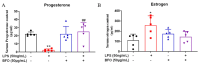
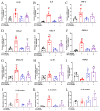
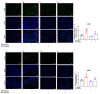
Most pharmacotherapeutic chemicals/interventions used to manage preterm labor (PTL) often cause neonatal morbidity and maternal adverse reactions. Fructooligosaccharides, extracted from traditional Chinese medicine, can alleviate inflammation, demonstrate antiviral capabilities, and protect against antioxidant stress, implying a potential effective PTL treatment. In this study, we explored the protective effects of the purified burdock fructooligosaccharide (BFO), a Gfn-type fructose polymer, on inflammation-induced PTL. It was found that two doses of 30 mg/kg mouse BFO administration to pregnant mice at a 6 h interval can effectively ameliorate lipopolysaccharide (LPS)-induced PTL. Drug dynamic distribution analysis revealed that BFO was rather highly enriched in myometrial tissues, could inhibit oxytocin-induced uterine smooth muscle contraction, and could bind toll-like receptor 4 (TLR4) on the membrane of uterine smooth muscle cells, downregulating the expression of downstream genes, attenuating the upregulation of inflammatory cytokines in serum and the myometrium, as well as reversing the increased macrophage and neutrophil infiltration into the myometrium induced by LPS. It can also interfere with the levels of estrogen and progesterone, alleviating the occurrence of premature birth. These findings collectively suggest that BFO might serve as a promising therapeutic agent for inflammation-related preterm labor to safeguard the health of both the mother and fetus.
Keywords: burdock fructooligosaccharide; inflammation; myometrium; preterm labor; toll-like receptor 4.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sharrow D., Hug L., You D., Alkema L., Black R., Cousens S., Croft T., Gaigbe-Togbe V., Gerland P., Guillot M., et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: A systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob. Health. 2022;10:e195–e206. doi: 10.1016/S2214-109X(21)00515-5. - DOI - PMC - PubMed
-
- Gomez-Lopez N., Galaz J., Miller D., Farias-Jofre M., Liu Z., Arenas-Hernandez M., Garcia-Flores V., Shaffer Z., Greenberg J.M., Theis K.R., et al. The immunobiology of preterm labor and birth: Intra-amniotic inflammation or breakdown of maternal–fetal homeostasis. Reproduction. 2022;164:R11–R45. doi: 10.1530/REP-22-0046. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2022YFC2704602/National Key Research and Development Project
- 2022YFC2704502/National Key Research and Development Project
- 82120108011/National Natural Science Foundation of China
- 82371699/National Natural Science Foundation of China
- 2021-01-07-00-07-E00144/Major Project of Shanghai Municipal Education Commission Scientific Research and Innovation Plan
LinkOut - more resources
Full Text Sources

