Plasma Exosomal-Derived SERPINA1 and GNAI2 Downregulation as Potential Diagnostic Biomarkers of Kawasaki Disease with Coronary Artery Aneurysms
- PMID: 40141310
- PMCID: PMC11942354
- DOI: 10.3390/ijms26062668
Plasma Exosomal-Derived SERPINA1 and GNAI2 Downregulation as Potential Diagnostic Biomarkers of Kawasaki Disease with Coronary Artery Aneurysms
Abstract
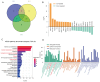
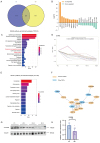
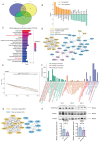
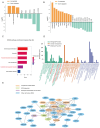
Kawasaki disease (KD) with coronary artery aneurysms (CAAs) is currently the primary cause of childhood acquired heart disease with an unclear pathogenesis. We established five groups for the discovery of differentially expressed proteins (DEPs): healthy control, febrile control, KD without CAAs, KD with small and medium CAAs, and KD with giant CAAs (n = 8 in each group). The validation of selected DEPs was conducted in another five groups (n = 4 in each group). We conducted comprehensive bioinformatics analyses to elucidate the functional roles of the DEPs in the groups of KD with CAAs and KD without CAAs. A total of 104 DEPs were identified in KD patients, which were primarily associated with complement-related pathways. A trend analysis of these 104 DEPs revealed 54 significantly changed DEPs associated with increased disease severity, which were primarily associated with G-protein-related functions. The alterations in α-1-antitrypsin short peptide (SERPINA1) and guanine nucleotide-binding protein G(i) subunit alpha-2 (GNAI2), which were selected from complement-related and G-protein-related pathways, respectively, were validated by Western blotting, and they were significantly decreased in KD patients with vs. without CAAs. In addition, we conducted an analysis of the DEPs in the groups of KD with CAAs and KD without CAAs, separately. There were 91 DEPs specifically expressed in KD patients with CAAs, associated with the neutrophil extracellular trap and complement pathways, while 16 DEPs were specific to those without CAAs, associated with viral infection and immunity pathways. Additionally, for DEPs among different severities of CAAs, there were 102 DEPs in KD patients with small and medium CAAs, associated with complement pathways and platelet activation pathways, whereas 34 DEPs were specific to giant CAAs, associated with the Rap1 signaling pathway and cell functions. In conclusion, this study provides plasmatic exosomal protein profiles in KD patients with CAAs, suggesting that SERPINA1 and GNIA2 might serve as novel potential diagnostic biomarkers for KD with CAAs.
Keywords: Kawasaki disease (KD); coronary artery aneurysms (CAAs); exosome; proteomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., Baker A.L., Jackson M.A., Takahashi M., Shah P.B., et al. Diagnosis, treatment, and long-term management of kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation. 2017;27:e927–e999. doi: 10.1161/CIR.0000000000000484. - DOI - PubMed
-
- Wu M.H., Chen H.C., Yeh S.J., Lin M.T., Huang S.C., Huang S.K. Prevalence and the long-term coronary risks of patients with kawasaki disease in a general population <40 years. Circ. Cardiovasc. Qual. Outcomes. 2012;5:566–570. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

