Developmental Endothelial Locus-1 Promotes Osteoclast Differentiation and Activation
- PMID: 40141315
- PMCID: PMC11942430
- DOI: 10.3390/ijms26062673
Developmental Endothelial Locus-1 Promotes Osteoclast Differentiation and Activation
Abstract
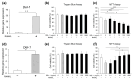
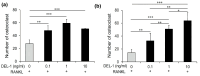
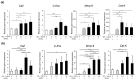
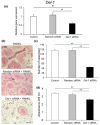
Developmental endothelial locus-1 (DEL-1) has traditionally been characterized within the scientific community as having anti-inflammatory properties with potential inhibitory effects on osteoclast formation. Our investigation challenges this paradigm by examining Del-1 expression in RAW264.7 cells and bone marrow-derived macrophages (BMMs) during osteoclastogenesis, as well as its functional impact on osteoclast development and activity. Our experimental findings revealed that Del-1 mRNA levels were markedly elevated in cells stimulated by the receptor activator of the nuclear factor κB ligand compared to unstimulated precursors. When cultured with varying concentrations of recombinant DEL-1, osteoclast differentiation increased in a dose-dependent manner. Furthermore, BMMs isolated from ovariectomized mice exhibited significantly higher Del-1 mRNA expression than those from control animals. To confirm DEL-1's role, we employed RNA interference techniques, demonstrating that DEL-1 silencing in RAW264.7 cells substantially reduced osteoclast formation. These results suggest that DEL-1 plays a previously unrecognized role in promoting osteoclastogenesis and may contribute to bone metabolism imbalances in conditions like osteoporosis, highlighting its complex role in skeletal homeostasis and its potential as a therapeutic target.
Keywords: DEL-1; bone metabolism; osteoclasts; osteoporosis.
Conflict of interest statement
Authors Keita Tachi, Hironori Kasai, and Ryutaro Shohara private practitioners and each one works at a private office. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Anti-IFN-γ Antibody Promotes Osteoclastogenesis in Human Bone Marrow Monocyte-Derived Macrophages Co-Cultured with Tuberculosis-Activated Th1 Cells.Cell Physiol Biochem. 2018;49(4):1512-1522. doi: 10.1159/000493455. Epub 2018 Sep 11. Cell Physiol Biochem. 2018. PMID: 30205409
-
Dominant negative N-cadherin inhibits osteoclast differentiation by interfering with beta-catenin regulation of RANKL, independent of cell-cell adhesion.J Bone Miner Res. 2005 Dec;20(12):2200-12. doi: 10.1359/JBMR.050809. Epub 2005 Aug 8. J Bone Miner Res. 2005. PMID: 16294273
-
WSS25, a sulfated polysaccharide, inhibits RANKL-induced mouse osteoclast formation by blocking SMAD/ID1 signaling.Acta Pharmacol Sin. 2015 Sep;36(9):1053-64. doi: 10.1038/aps.2015.65. Epub 2015 Aug 24. Acta Pharmacol Sin. 2015. PMID: 26299951 Free PMC article.
-
Docosahexaenoic acid signaling attenuates the proliferation and differentiation of bone marrow-derived osteoclast precursors and promotes apoptosis in mature osteoclasts.Cell Signal. 2017 Jan;29:226-232. doi: 10.1016/j.cellsig.2016.11.007. Epub 2016 Nov 9. Cell Signal. 2017. PMID: 27836739
-
RNA-binding protein Musashi2 induced by RANKL is critical for osteoclast survival.Cell Death Dis. 2016 Jul 21;7(7):e2300. doi: 10.1038/cddis.2016.213. Cell Death Dis. 2016. PMID: 27441652 Free PMC article.
References
-
- Vernal R., Dutzan N., Chaparro A., Puente J., Valenzuela M.A., Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J. Clin. Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

