Prunin: An Emerging Anticancer Flavonoid
- PMID: 40141319
- PMCID: PMC11942023
- DOI: 10.3390/ijms26062678
Prunin: An Emerging Anticancer Flavonoid
Abstract
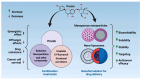
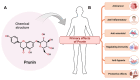
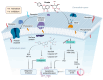
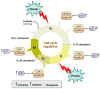
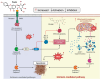
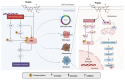
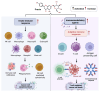
Despite the substantial advances in cancer therapies, developing safe and effective treatment methodologies is critical. Natural (plant-derived compounds), such as flavonoids, might be crucial in developing a safe treatment methodology without toxicity toward healthy tissues. Prunin is a flavonoid with the potential to be used in biomedical applications. Prunin has yet to undergo thorough scientific research, and its precise molecular mechanisms of action remain largely unexplored. This review summarizes the therapeutic potential of prunin for the first time, focusing on its underlying mechanisms as an anticancer compound. Prunin has gained significant attention due to its antioxidant, anti-inflammatory, and anticancer effects. This review aims to unlock how prunin functions at the molecular level to exert its anticancer effects, primarily modulating key cellular pathways. Furthermore, we have discussed the prunin's potential as an adjunctive therapy with conventional treatments, highlighting its ability to strengthen treatment responses while decreasing drug resistance. Moreover, the discussion probes into innovative delivery methods, particularly nanoformulations, that might address prunin's bioavailability, solubility, and stability limitations and optimize its therapeutic application. By providing a comprehensive analysis of prunin's properties, this review aims to stimulate further exploration of using prunin as an anticancer agent, thereby progressing the development of targeted, selective, safe, and effective therapeutic methods.
Keywords: anticancer; combination therapy; flavonoid; nanoformulation; natural compounds; prunin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Tzanova M., Atanasov V., Yaneva Z., Ivanova D., Dinev T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes. 2020;8:1222. doi: 10.3390/pr8101222. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

