Tackling Prostate Cancer with Theranostic E5B9-Bombesin Target Modules (TMs): From Imaging to Treatment with UniCAR T-Cells
- PMID: 40141329
- PMCID: PMC11941939
- DOI: 10.3390/ijms26062686
Tackling Prostate Cancer with Theranostic E5B9-Bombesin Target Modules (TMs): From Imaging to Treatment with UniCAR T-Cells
Abstract
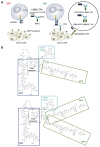
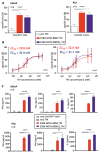
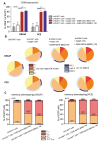
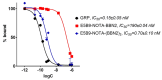
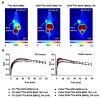
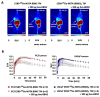
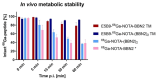
Target modules (TMs), intermediate molecules required for UniCAR T-cell therapy, are promising molecules for immunotheranostic approaches. In the current work, we developed TMs containing a monomeric or dimeric form of the antagonist bombesin peptide (BBN2) and assessed their potential for diagnostic imaging using positron emission tomography (PET) as well as immunotherapy in combination with UniCAR T-cells to target and image GRPR expression in prostate cancer. Synthesized monomeric and dimeric BBN2 TMs retained binding to GRPR in vitro. Both BBN2 TMs specifically activated and redirected UniCAR T-cells to eradicate PC3 and LNCaP cancer cells with high efficiency and in a comparable manner. UniCAR T-cells retained a non-exhausted memory phenotype favorable to their persistence and fitness. The 68Ga-labeled BBN2 TMs showed proof-of-target towards GRPR in PC3 and LNCaP xenografts with similar uptake profiles for both BBN2 TMs in dynamic PET experiments. Clearance occurred exclusively through renal elimination. A tremendously increased in vivo metabolic stability of the BBN2 TMs was observed compared to their counterparts without E5B9. Both monomeric and dimeric BBN2 TMs represent novel and promising immunotheranostic tools for application in prostate cancer with exceptionally high in vivo metabolic stability.
Keywords: PET; UniCAR T-cell therapy; bombesin; prostate cancer; theranostics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Dagar G., Gupta A., Masoodi T., Nisar S., Merhi M., Hashem S., Chauhan R., Dagar M., Mirza S., Bagga P., et al. Harnessing the potential of CAR-T cell therapy: Progress, challenges, and future directions in hematological and solid tumor treatments. J. Transl. Med. 2023;21:449. doi: 10.1186/s12967-023-04292-3. - DOI - PMC - PubMed
-
- Koristka S., Cartellieri M., Feldmann A., Arndt C., Loff S., Michalk I., Aliperta R., von Bonin M., Bornhäuser M., Ehninger A., et al. Flexible Antigen-Specific Redirection of Human Regulatory T Cells Via a Novel Universal Chimeric Antigen Receptor System. Blood. 2014;124:3494. doi: 10.1182/blood.V124.21.3494.3494. - DOI
-
- Cartellieri M., Feldmann A., Koristka S., Arndt C., Loff S., Ehninger A., von Bonin M., Bejestani E.P., Ehninger G., Bachmann M.P. Switching CAR T cells on and off: A novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6:e458. doi: 10.1038/bcj.2016.61. - DOI - PMC - PubMed
-
- Loureiro L.R., Feldmann A., Bergmann R., Koristka S., Berndt N., Arndt C., Pietzsch J., Novo C., Videira P., Bachmann M. Development of a novel target module redirecting UniCAR T cells to Sialyl Tn-expressing tumor cells. Blood Cancer J. 2018;8:81. doi: 10.1038/s41408-018-0113-4. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

