Activin A Inhibitory Peptides Suppress Fibrotic Pathways by Targeting Epithelial-Mesenchymal Transition and Fibroblast-Myofibroblast Transformation in Idiopathic Pulmonary Fibrosis
- PMID: 40141346
- PMCID: PMC11942258
- DOI: 10.3390/ijms26062705
Activin A Inhibitory Peptides Suppress Fibrotic Pathways by Targeting Epithelial-Mesenchymal Transition and Fibroblast-Myofibroblast Transformation in Idiopathic Pulmonary Fibrosis
Abstract
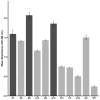
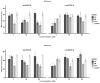
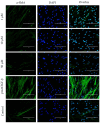
Idiopathic pulmonary fibrosis (IPF) is a progressive and incurable chronic interstitial lung disease characterized by excessive fibrosis and impaired lung function. Current treatments, such as pirfenidone and nintedanib, slow disease progression but fail to halt or reverse fibrosis, highlighting the need for novel approaches. Activin A, which belongs to the TGF-β superfamily, is implicated in various fibrosis-related mechanisms, including epithelial-mesenchymal transition (EMT), a process where epithelial cells acquire mesenchymal characteristics, and fibroblast-myofibroblast transformation (FMT), in which fibroblasts differentiate into contractile myofibroblasts. It also promotes inflammatory cytokine release and extracellular matrix buildup. This study aimed to inhibit Activin A activity using synthetic peptides identified through phage display screening. Of the ten peptides isolated, A7, B9, and E10 demonstrated high binding affinity and inhibitory activity. Computational modeling confirmed that these peptides target the receptor-binding domain of Activin A, with peptide E10 exhibiting superior efficacy. Functional assays showed that E10 reduced cell migration, inhibited EMT in A549 cells, and suppressed FMT in fibroblast cultures, even under pro-fibrotic stimulation with TGF-β. These findings underscore the therapeutic potential of targeting Activin A with synthetic peptides, offering a promising avenue for IPF treatment and expanding the arsenal of anti-fibrotic strategies.
Keywords: Activin A; anti-fibrotic therapy; epithelial–mesenchymal transition; fibroblast–myofibroblast transformation; idiopathic pulmonary fibrosis; synthetic peptides.
Conflict of interest statement
The authors state that they have no conflicts of interest. The funding sources played no part in the study’s design, data collection, analysis, or interpretation, nor in the writing of the manuscript or the decision to publish the findings.
Figures




References
-
- George P.M., Spagnolo P., Kreuter M., Altinisik G., Bonifazi M., Martinez F.J., Molyneaux P.L., Renzoni E.A., Richeldi L., Tomassetti S., et al. Progressive Fibrosing Interstitial Lung Disease: Clinical Uncertainties, Consensus Recommendations, and Research Priorities. Lancet Respir. Med. 2020;8:925–934. doi: 10.1016/S2213-2600(20)30355-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

