Prolonged Low-Dose Administration of FDA-Approved Drugs for Non-Cancer Conditions: A Review of Potential Targets in Cancer Cells
- PMID: 40141362
- PMCID: PMC11942989
- DOI: 10.3390/ijms26062720
Prolonged Low-Dose Administration of FDA-Approved Drugs for Non-Cancer Conditions: A Review of Potential Targets in Cancer Cells
Abstract
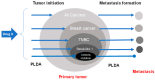
Though not specifically designed for cancer therapy, several FDA-approved drugs such as metformin, aspirin, and simvastatin have an effect in lowering the incidence of cancer. However, there is a great discrepancy between in vitro concentrations needed to eliminate cancer cells and the plasma concentration normally tolerated within the body. At present, there is no universal explanation for this discrepancy and several mechanisms have been proposed including targeting cancer stem cells (CSCs) or cellular senescence. CSCs are cells with the ability of self-renewal and differentiation known to be resistant to chemotherapy. Senescence is a response to damage and stress, characterized by permanent cell-cycle arrest and apoptotic resistance. Although, for both situations, there are few examples where low concentrations of the FDA-approved drugs were the most effective, there is no satisfactory data to support that either CSCs or cellular senescence are the target of these drugs. In this review, we concisely summarize the most used FDA-approved drugs for non-cancer conditions as well as their potential mechanisms of action in lowering cancer incidence. In addition, we propose that prolonged low-dose administration (PLDA) of specific FDA-approved drugs can be useful for effectively preventing metastasis formation in selected patients.
Keywords: carcinogenesis metastasis; chemoprevention; plasticity; senescence; stem cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

