Cultured Macrophage Models for the Investigation of Lysosomal Glucocerebrosidase and Gaucher Disease
- PMID: 40141367
- PMCID: PMC11943430
- DOI: 10.3390/ijms26062726
Cultured Macrophage Models for the Investigation of Lysosomal Glucocerebrosidase and Gaucher Disease
Abstract
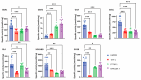
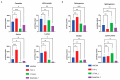
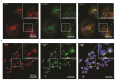
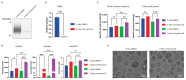
Macrophages are specialised cells that degrade a range of substrates during their lifetime. In inherited lysosomal storage disorders, particularly the sphingolipidoses, macrophages transform into storage cells and contribute to pathology. An appropriate cultured macrophage model is desired for fundamental research and the assessment of considered therapeutic interventions. We compared commonly used macrophage cell lines, RAW264.7, J774A.1, and THP-1 cells, with human monocyte-derived macrophages (HMDMs) isolated from peripheral blood. Specific lysosomal glucosidases were analysed by enzymatic activity measurements and visualised with fluorescent activity-based probes. Special attention was given to lysosomal glucocerebrosidase (GBA1), the enzyme deficient in Gaucher disease in which lipid-laden macrophages are a hallmark. In macrophage cell lines and HMDMs, various (glyco)sphingolipids relevant to GBA1 activity were determined. Finally, the feasibility of inactivation of GBA1 with a cell-permeable suicide inhibitor was established, as well as the monitoring of uptake of therapeutic recombinant human GBA1. Major differences among various cell lines were noted in terms of morphology, lysosomal enzyme expression, and glycosphingolipid content. HMDMs appear to be the most suitable model for investigations into GBA1 and Gaucher disease. Moreover, they serve as a valuable model for mannose-receptor mediated uptake of therapeutic human GBA1, effectively mimicking enzyme replacement therapy for Gaucher disease.
Keywords: Gaucher disease; glucocerebrosidase; lysosome; macrophage; monocyte.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

