Immunotherapies Targeting CD123 and CD303: A New Frontier in Treating Blastic Plasmacytoid Dendritic Cell Neoplasm
- PMID: 40141368
- PMCID: PMC11942551
- DOI: 10.3390/ijms26062732
Immunotherapies Targeting CD123 and CD303: A New Frontier in Treating Blastic Plasmacytoid Dendritic Cell Neoplasm
Abstract
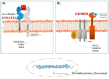
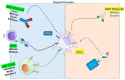
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematologic malignancy characterized by the overexpression of CD123 and CD303 surface antigens. These molecular markers play a crucial role in diagnosing diseases and developing targeted therapies. Traditional treatment options for BPDCN have demonstrated limited effectiveness, highlighting the need for new and innovative therapeutic strategies. Recent advances in immunotherapy, particularly therapeutic monoclonal antibodies, bispecific T-cell engagers, and CAR T-cell therapy, have provided promising alternatives. Tagraxofusp, the first FDA-approved CD123-targeted therapy, has significantly improved patient outcomes. Additionally, emerging CD303-targeting strategies offer the potential for further advancements. Despite these breakthroughs, challenges such as treatment resistance and toxicity remain. This review explores the latest developments in BPDCN treatment, emphasizing the potential of CD123 and CD303 as targets for precision medicine interventions. The ongoing evolution of targeted immunotherapies holds promise for improving patient survival and redefining treatment paradigms in hematologic malignancies.
Keywords: BPDCN; CD123; CD303; cancer therapy; immunotherapy; pDC.
Conflict of interest statement
A.P. declares lectures honoraria from Hoffmann-La Roche AG, Incyte-Italy, Merck Sharp and Dohme, and Bristol-Meyers Squibb, and participation on scientific advisory boards for F. Hoffmann-La Roche AG, Merck Sharp and Dohme, Takeda, and Incyte-Italy. All other authors have declared no conflicts of interest.
Figures

References
-
- Pagano L., Valentini C.G., Pulsoni A., Fisogni S., Carluccio P., Mannelli F., Lunghi M., Pica G., Onida F., Cattaneo C., et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: An Italian multicenter study. Haematologica. 2013;98:239–246. doi: 10.3324/haematol.2012.072645. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

